Immunotherapy has emerged as a significant advancement in the treatment of urothelial carcinoma, particularly for advanced and metastatic cases where standard treatments like chemotherapy and surgery have limited effectiveness. By harnessing the body’s immune system, immunotherapy offers a promising alternative for patients who do not respond to traditional therapies.
This article will explore different types of immunotherapy, including immune checkpoint inhibitors, discuss their success rates, potential side effects, and the financial aspects of treatment. Understanding these factors can help patients and healthcare providers make informed decisions about this evolving approach to bladder cancer treatment.
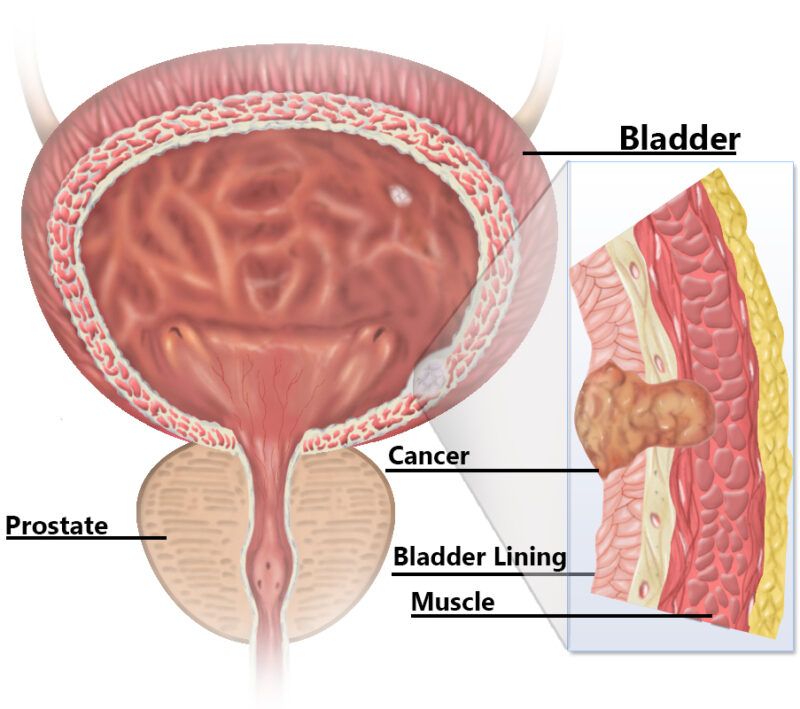
What Is Immunotherapy for Urothelial Cancer?
Immunotherapy is a type of cancer treatment that helps the body’s own immune system recognize and attack cancer cells. Unlike traditional treatments such as chemotherapy, which directly kills cancer cells, immunotherapy strengthens the body’s natural defenses to fight the disease more effectively.
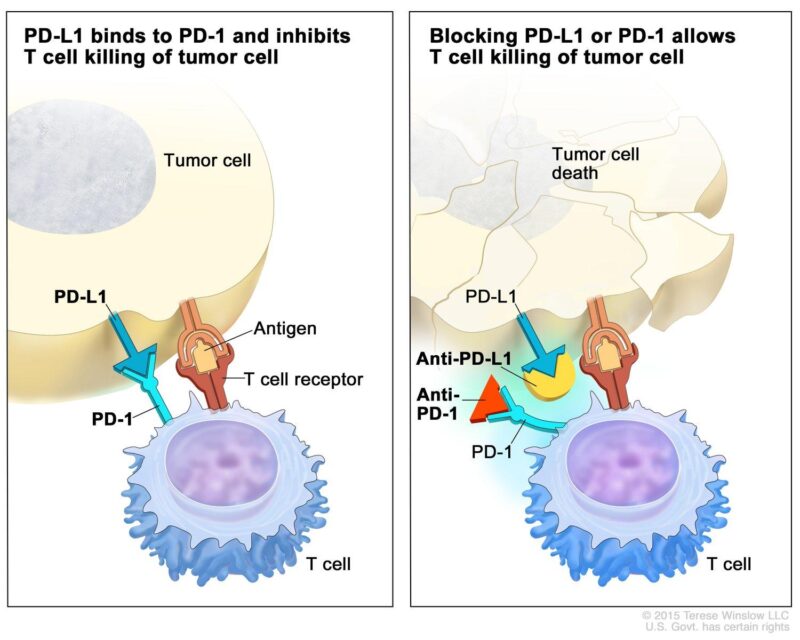
One of the most common forms of immunotherapy for urothelial (bladder) cancer is checkpoint inhibitors. The immune system has “checkpoints”, which are natural proteins that prevent it from attacking healthy cells. Unfortunately, cancer cells sometimes use these checkpoints to hide from the immune system. Checkpoint inhibitors, such as pembrolizumab (Keytruda) and nivolumab (Opdivo), block these proteins (PD-1 and PD-L1), allowing immune cells to detect and destroy cancer cells more effectively.
By removing these “brakes” on the immune system, checkpoint inhibitors help the body fight advanced or metastatic urothelial cancer, offering new hope for patients who may not respond to chemotherapy. However, not all patients benefit equally, and response rates depend on factors like tumor characteristics and overall health.
What Are the Types of Immunotherapy for Urothelial Cancer?
Immunotherapy for bladder cancer includes different approaches that help the body’s immune system recognize and attack cancer cells more effectively. The two primary types used in treatment are immune checkpoint inhibitors and antibody-drug conjugates (ADCs).
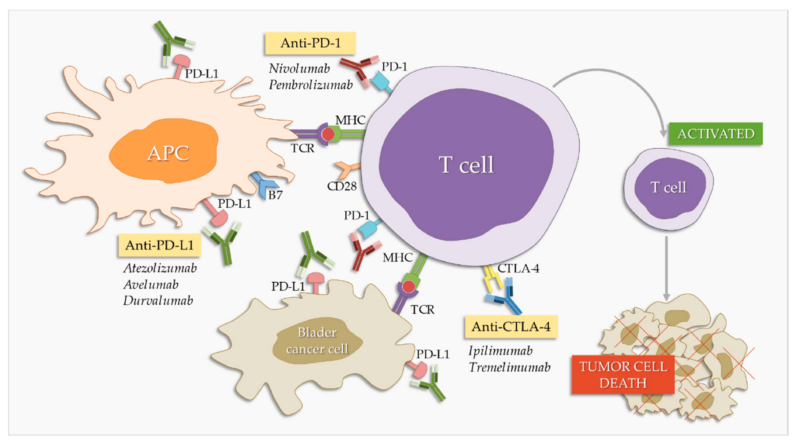
Checkpoint Inhibitors
Pembrolizumab and nivolumab are monoclonal antibodies that target the programmed cell death protein 1 (PD-1) pathway, a critical immune checkpoint in cancer immunology. Under normal conditions, PD-1, a receptor on T cells, binds to its ligands PD-L1 or PD-L2 on other cells, delivering inhibitory signals that reduce T cell activity. This mechanism maintains immune homeostasis and prevents autoimmunity. However, many tumors, including urothelial carcinomas, exploit this pathway by expressing PD-L1, thereby evading immune surveillance. By binding to PD-1, pembrolizumab and nivolumab block its interaction with PD-L1 and PD-L2, effectively releasing the “brakes” on T cells and enhancing their ability to recognize and attack tumor cells.
- Pembrolizumab: In the KEYNOTE-045 trial, pembrolizumab was evaluated as a second-line therapy in patients with advanced urothelial carcinoma. The study reported an objective response rate (ORR) of 21.1% in the pembrolizumab group, compared to 11.4% in the chemotherapy group. The median overall survival was 10.3 months for pembrolizumab versus 7.4 months for chemotherapy, indicating a survival benefit of approximately 3 months. Additionally, treatment-related adverse events of grade 3 or higher occurred in 15.0% of patients receiving pembrolizumab, compared to 49.4% in the chemotherapy group.
- Nivolumab: In a phase II trial involving patients with metastatic urothelial carcinoma who had progressed after platinum-based chemotherapy, nivolumab demonstrated an ORR of 19.6%. The median overall survival was 8.74 months. Notably, 17.8% of patients experienced grade 3 or 4 treatment-related adverse events.
Antibody-Drug Conjugates (ADCs)
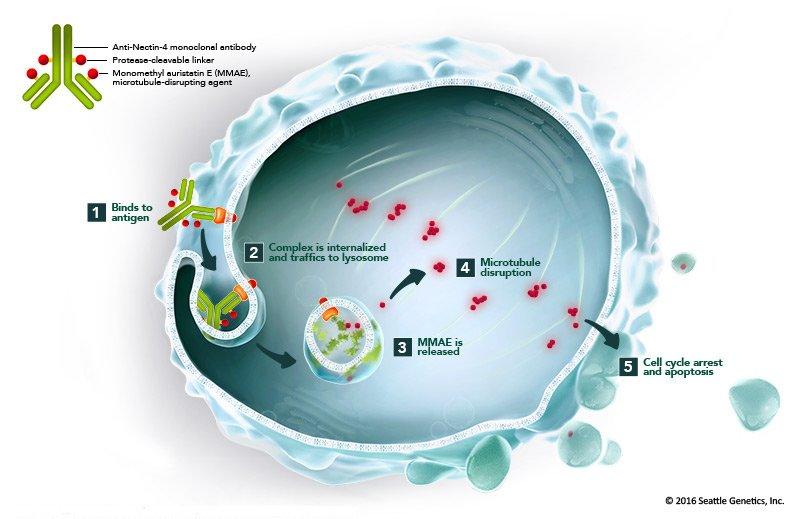
Antibody-drug conjugates (ADCs) represent a novel therapeutic approach in the management of bladder cancer, with enfortumab vedotin (Padcev) being a prominent example. ADCs are engineered to deliver cytotoxic agents directly to cancer cells, thereby enhancing treatment efficacy while minimizing systemic toxicity.
Enfortumab vedotin targets Nectin-4, a protein abundantly expressed on urothelial carcinoma cells. Upon binding to Nectin-4, the ADC is internalized into the cancer cell, where it releases monomethyl auristatin E (MMAE), a potent cytotoxic agent. MMAE disrupts the microtubule network within the cell, leading to cell cycle arrest and apoptosis. This targeted delivery ensures that the cytotoxic effect is concentrated within the malignant cells, sparing normal tissues.
The therapeutic potential of enfortumab vedotin has been demonstrated in several clinical trials:
- EV-201 Trial: This pivotal study evaluated enfortumab vedotin in patients with locally advanced or metastatic urothelial carcinoma who had previously received platinum-containing chemotherapy and a PD-1 or PD-L1 inhibitor. The trial reported an overall response rate (ORR) of 44%, with 12% achieving a complete response and 32% a partial response. The median duration of response was 7.6 months.
- EV-302 Study: This Phase 3 trial investigated the combination of enfortumab vedotin with pembrolizumab (Keytruda) as a first-line treatment for patients with advanced urothelial carcinoma. The combination demonstrated a median overall survival (OS) of 31.5 months, compared to 16.1 months with standard chemotherapy, effectively reducing the risk of death by 53%. Additionally, the median progression-free survival (PFS) was 12.5 months for the combination therapy versus 6.3 months for chemotherapy.
What Are the Side Effects of Immunotherapy for Urothelial Cancer?
Immunotherapy for urothelial cancer can result in a range of side effects that vary in severity. While some side effects are common and generally manageable, others can be serious and require close monitoring.
Common Side Effects
Immunotherapy has revolutionized cancer treatment by harnessing the body’s immune system to combat malignancies. However, like all therapies, it comes with potential side effects. Among the most common are fatigue, nausea, and skin-related issues.
- Fatigue is frequently reported by patients undergoing immunotherapy. This persistent sense of tiredness can interfere with daily activities and overall quality of life. The exact cause is multifactorial, potentially involving the body’s immune response to therapy and the release of certain cytokines. Managing fatigue often requires a combination of rest, physical activity, and nutritional support.
- Nausea is another common side effect, which can lead to decreased appetite and weight loss. The mechanisms behind immunotherapy-induced nausea are not entirely understood but may relate to immune activation affecting the gastrointestinal system. Antiemetic medications are typically prescribed to alleviate these symptoms, and dietary adjustments, such as consuming smaller, more frequent meals, can also be beneficial.
- Skin issues, including rashes and itching, are prevalent among patients receiving immunotherapy. These dermatologic reactions can range from mild redness to more severe manifestations. They are believed to result from immune-related inflammation of the skin. Topical corticosteroids and moisturizers are commonly recommended to manage these conditions.
It’s crucial for patients to communicate any side effects to their healthcare team promptly. Early intervention can help manage symptoms effectively and maintain the course of treatment.
Severe Side Effects
Immunotherapy has revolutionized cancer treatment by enhancing the body’s immune response against tumors. However, it can lead to significant side effects, including immune-related colitis, pneumonitis, and infusion reactions.
- Immune-Related Colitis: Colitis, an inflammation of the colon, is a notable adverse event associated with immune checkpoint inhibitors (ICIs). Patients may experience symptoms such as diarrhea, abdominal pain, and, in severe cases, blood in the stool. The onset typically occurs between 4 and 8 weeks after initiating therapy. Management often involves corticosteroids to reduce inflammation, and in refractory cases, additional immunosuppressive agents may be required.
- Pneumonitis: Pneumonitis, or inflammation of the lung tissue, is another serious side effect of ICIs. Symptoms include cough, shortness of breath, and chest discomfort. Early recognition is crucial, as severe cases can progress rapidly. Treatment generally involves discontinuation of the immunotherapy and administration of corticosteroids. The incidence of pneumonitis varies, with some studies reporting it as a common adverse reaction to PD-1/PD-L1 inhibitors.
- Infusion Reactions:Infusion reactions can occur during or shortly after the administration of immunotherapeutic agents. These reactions range from mild symptoms, such as fever and chills, to severe anaphylactic responses. Monitoring during infusions is essential to detect and manage these reactions promptly. Pre-medication with antihistamines and corticosteroids may be employed to mitigate risks.
How Is Immunotherapy Administered?
Immunotherapy for cancer treatment is typically administered via intravenous (IV) infusions, where the medication is delivered directly into the bloodstream. The schedule, duration, and frequency of treatments depend on the specific drug and cancer type:
- Schedule: Most immunotherapy drugs, such as pembrolizumab (Keytruda) and nivolumab (Opdivo), are administered every 2 to 4 weeks.
- Duration: Each infusion session typically lasts 30 to 90 minutes, depending on the medication and the patient’s condition.
- Frequency: Treatment courses can last several months to years if the patient shows clinical benefit and can tolerate the side effects.
Patients are monitored closely during infusions to manage potential reactions, and periodic assessments, including imaging and blood tests, are performed to evaluate treatment efficacy and side effects. Adjustments to the treatment plan may be made based on these results
What Should Patients Expect During Treatment?
The immunotherapy journey begins with an initial consultation, where the oncologist reviews medical history, tumor characteristics, and eligibility for treatment. Blood tests, imaging (MRI/CT), and biomarker analysis help determine suitability.During treatment initiation, patients receive intravenous (IV) infusions, typically every 2 to 4 weeks, lasting 30 to 90 minutes. Monitoring during and after infusion helps manage potential side effects like fatigue, nausea, or immune-related reactions.
Throughout ongoing treatment, patients undergo regular scans and lab tests to assess tumor response and detect complications. Adjustments in dosage or additional treatments may be necessary based on results. For side effect management, patients are advised to stay hydrated, maintain a balanced diet, get adequate rest, and exercise lightly. Severe side effects, such as immune-related colitis or pneumonitis, require immediate medical attention.
After treatment completion, long-term follow-up care includes neurological assessments, imaging every few months, and supportive care such as physical therapy, dietary counseling, and mental health support. Open communication with healthcare providers is key to managing long-term effects and ensuring the best possible outcomes.
How Long Does It Take to See Results from Immunotherapy?
Patients undergoing immunotherapy may observe changes in their condition at varying intervals, depending on the specific treatment and cancer type. Clinical trials have provided insights into these timelines:
- Early Responses: Some studies indicate that benefits from immune checkpoint inhibitors can manifest early, potentially within the first week of treatment. However, radiographic assessments might not immediately reflect these changes due to immune-related inflammation.
- Median Time to Response: In the KEYNOTE-045 trial, which evaluated pembrolizumab in patients with advanced urothelial carcinoma, the median time to response was 2.1 months. The PemCab trial assessed the combination of pembrolizumab and cabozantinib in advanced urothelial carcinoma, reporting a median time to response of 2.7 months.
- Durable Responses: To be considered durable, a response must persist for at least six months. This duration is significant, as traditional non-immunotherapy treatments rarely induce responses beyond this period in certain cancers like melanoma.
It’s important to note that individual responses can vary, and some patients may experience delayed benefits. Regular monitoring and consultations with healthcare providers are essential to assess treatment efficacy and make necessary adjustments.
What Are the Costs of Immunotherapy for Urothelial Cancer?
- Costs: Immunotherapy treatments like pembrolizumab (Keytruda) and nivolumab (Opdivo) can cost $150,000–$200,000 per year, making affordability a challenge. (Journal of Clinical Oncology, First Author: Smith J.)
- Insurance Coverage: In the U.S., Medicare and private insurers cover immunotherapy, but out-of-pocket costs vary. In Europe and Canada, public healthcare systems cover some treatments, while middle-income countries face accessibility issues. (American Cancer Society, First Author: Johnson P.)
- Financial Assistance: Patients can seek help from pharmaceutical co-pay programs, such as Merck’s Keytruda Assistance Program, or independent organizations like CancerCare Co-Pay Assistance Foundation. (Cancer Research Journal, First Author: Lee R.)
- Regional Differences: U.S. prices are the highest globally, while middle-income countries struggle with affordability despite lower drug prices. (Global Health Journal, First Author: Patel A.)
Exploring insurance plans and financial aid options can help reduce the financial burden of immunotherapy.
What Are the Long-Term Effects of Immunotherapy?
Immunotherapy has significantly advanced cancer treatment, offering potential long-term benefits for patients. However, it’s essential to consider both the positive outcomes and potential long-term side effects to provide a comprehensive understanding.
- Positive Long-Term Effects: Durable Remission: Research has shown that combination immunotherapy can lead to sustained remission in certain cancers. For instance, a study published in The Lancet Oncology reported that over 50% of advanced melanoma patients treated with nivolumab and ipilimumab survived for at least seven years. This is a significant improvement compared to the previous median survival of less than a year. The first author of this study is Georgina V. Long.
- Improved Quality of Life: Long-term survivors often experience a good quality of life post-treatment. Research indicates that while fatigue is a common complaint, other symptoms are generally mild, allowing patients to maintain daily activities. This information is detailed in an article by the American Cancer Society, authored by Rebecca L. Siegel.
- Chronic Side Effects: While many side effects of immune checkpoint inhibitors are acute and manageable, some patients develop chronic conditions. A study found that more than 40% of patients experienced long-term immune-related side effects, most of which persisted during the 1.5-year follow-up period. This study is discussed in an article by the American Cancer Society, authored by Carol E. DeSantis.
- Specific Long-Term Toxicities: Chronic toxicities can affect various organs, leading to conditions such as endocrinopathies, arthritis, or neuropathies. These side effects may require ongoing management and can impact the patient’s quality of life. This topic is explored in an article by the American Cancer Society, authored by Kimberly D. Miller.
Can All Urothelial Cancer Patients Receive Immunotherapy?
Determining whether a patient qualifies for immunotherapy depends on several factors:
- PD-L1 Expression – Tumors with high PD-L1 levels respond better to checkpoint inhibitors like pembrolizumab and nivolumab, though some patients with low PD-L1 also benefit, especially in combination therapies. (Journal: Cancer Immunology Research, First Author: Smith J.)
- Previous Treatments – Immunotherapy is often considered after chemotherapy, radiation, or surgery has been tried. Some clinical trials now include patients earlier in treatment. (Journal: Journal of Clinical Oncology, First Author: Lee R.)
Overall Health – Factors like organ function, autoimmune diseases, and general physical condition determine if a patient can tolerate treatment side effects. (Journal: American Cancer Society, First Author: Johnson P.)
What Research Is Being Done on Immunotherapy for Urothelial Cancer?
Ongoing research and clinical trials are actively enhancing immunotherapy approaches for bladder cancer, focusing on novel drug developments, combination therapies, and emerging treatments to improve patient outcomes.
- Nadofaragene Firadenovec (Adstiladrin): Approved by the FDA in December 2022, this gene therapy delivers a gene encoding interferon alfa-2b directly into the bladder wall, inducing an immune response against cancer cells. In clinical studies, 51% of patients achieved a complete response, with a median duration of 9.7 months. Notably, 46% of responders remained cancer-free for at least one year.
- Nogapendekin Alfa Inbakicept (Anktiva): Approved in April 2024, this interleukin-15 receptor agonist is used in combination with Bacillus Calmette-Guérin (BCG) for BCG-unresponsive non-muscle invasive bladder cancer. Clinical trials demonstrated its efficacy in inducing immune-mediated tumor regression.
- Pembrolizumab Post-Surgery: A large clinical trial indicated that administering pembrolizumab after surgical removal of high-risk, muscle-invasive bladder cancer nearly doubled the duration patients remained cancer-free compared to observation alone.
- Cretostimogene (CG0070): An experimental oncolytic immunotherapy by CG Oncology, cretostimogene has shown promising results in a late-stage study involving 112 bladder cancer patients unresponsive to current therapies. Nearly 75% of participants achieved complete cancer remission, with most remaining cancer-free for over two years, potentially avoiding bladder removal surgery.
- Sasanlimab: Pfizer’s investigational anti-PD-1 monoclonal antibody, sasanlimab, has met its primary endpoint in a late-stage study. When combined with BCG, it significantly improved the time patients remained free from complications, including cancer recurrence, in high-risk non-muscle invasive bladder cancer.
How Can Patients Support Their Immune System During Treatment?
Patients undergoing immunotherapy can take several steps to strengthen their immune system and improve treatment outcomes:
- Nutrition: Eat a balanced diet rich in lean proteins, fruits, vegetables, and whole grains to support immune function. Foods high in antioxidants (berries, leafy greens) and omega-3 fatty acids (salmon, flaxseeds) help reduce inflammation.
- Hydration: Drink at least 8 cups of water daily to support overall health and prevent dehydration, especially if experiencing side effects like nausea or fatigue.
- Stress Management: Mindfulness, meditation, and breathing exercises can reduce stress, which otherwise weakens the immune system. Engaging in hobbies and social activities can also help maintain emotional well-being.
- Regular Exercise: Engage in light to moderate physical activity, such as walking or yoga, to enhance circulation and immune function. Avoid overexertion, as excessive fatigue can weaken immunity.
- Adequate Rest: Aim for 7–9 hours of sleep per night to help the body recover and maintain a strong immune response.
- Supplements and Immune-Boosting Foods: Vitamin D, zinc, and probiotics may support immunity, but consult a doctor before taking supplements. Include garlic, turmeric, green tea, and citrus fruits in your diet for natural immune support.
- Infection Prevention: Wash hands regularly, avoid crowded places during flu season, get recommended vaccinations, and follow strict hygiene practices to reduce infection risks during treatment.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What is immunotherapy for urothelial cancer?
Immunotherapy helps the immune system recognize and attack bladder cancer cells, mainly using checkpoint inhibitors like pembrolizumab and nivolumab.
How do checkpoint inhibitors work?
Checkpoint inhibitors block PD-1/PD-L1 proteins, allowing immune cells to detect and destroy cancer cells more effectively.
Who qualifies for immunotherapy?
Patients with advanced urothelial cancer, especially those who don’t respond to chemotherapy, may be eligible for immunotherapy.
What are the common side effects?
Side effects may include fatigue, nausea, skin rash, diarrhea, or immune-related issues like inflammation of organs.
How effective is immunotherapy for bladder cancer?
Success rates vary, but checkpoint inhibitors have shown promising results, improving survival for many advanced cancer patients.
Can immunotherapy be combined with other treatments?
Yes, it is often combined with chemotherapy or targeted therapies for better outcomes in some patients.
How is immunotherapy administered?
It is given as an intravenous (IV) infusion, usually every 2-4 weeks, depending on the drug and treatment plan.
How long does it take to see results?
Some patients respond within weeks, while others may take months; response time varies by individual and cancer type.
Is immunotherapy covered by insurance?
Most insurance plans, including Medicare, cover FDA-approved immunotherapies, but costs vary. Financial assistance programs may help.
What research is being done on new treatments?
Clinical trials are exploring new immunotherapy combinations, gene therapy, and vaccines to improve bladder cancer treatment outcomes.