Colon cancer with BRAF V600E mutations represents a biologically aggressive subset, occurring in roughly 8%–10% of cases and often presenting with right-sided tumors, older age at diagnosis, and poor responses to standard chemotherapy. While microsatellite instability–high (MSI-H) tumors can derive substantial benefit from PD-1 blockade, nearly 80% of BRAF V600E colorectal cancers are microsatellite stable (MSS) and remain largely unresponsive to immunotherapy alone.
Background
The combination of the BRAF inhibitor encorafenib with the anti-EGFR antibody cetuximab is an approved regimen for refractory BRAF V600E metastatic colorectal cancer (mCRC). In the phase 3 BEACON trial, encorafenib plus cetuximab achieved a median progression-free survival (PFS) of 4.2 months, median overall survival (OS) of 9.4 months, and an overall response rate (ORR) of 20%, with disease control that was still relatively short-lived. Preclinical and translational data suggest that BRAFV600E MSS tumors display greater immune activation than BRAF wild-type MSS tumors, and that MAPK inhibition may enhance anti-tumor immunity.
The current phase 1/2 study (NCT04017650) tested whether adding nivolumab to encorafenib and cetuximab could improve clinical outcomes in this high-risk population and sought to identify transcriptomic biomarkers of response using both tumor tissue and extracellular vesicle RNA (evRNA) from plasma.
Methods
This was a single-institution, phase 1/2 trial in adults with unresectable or metastatic adenocarcinoma of the colon or rectum harboring a confirmed BRAFV600E mutation and MSS/proficient mismatch repair status in a CLIA-certified laboratory. Patients had received one or two prior systemic regimens for metastatic disease, had ECOG performance status 0–1, adequate organ function, and no prior exposure to BRAF/MEK/ERK inhibitors, anti-EGFR antibodies, or immune checkpoint inhibitors.
In the phase 1 component, a dose de-escalation design was used to determine a safe dose level for the triplet combination, focusing on dose-limiting toxicities (DLTs) during the first 28-day cycle. The phase 2 component followed a Simon two-stage design with a null hypothesis that ORR ≤22% and an alternative hypothesis that ORR ≥45%. At least 4 of the first 15 patients had to respond to proceed to full enrollment; the regimen would be considered promising if ≥10 of 26 treated patients achieved a radiographic response.
Exploratory objectives included deep molecular characterization of baseline tumor biopsies and serial plasma samples. Bulk RNA sequencing of pretreatment tumor tissue and evRNA sequencing from plasma were used to explore pathway-level signatures (MAPK activation, immune activation, complement, metabolic pathways) associated with response or resistance.
Study Design
All patients received the same “dose level 1” schedule, which was declared safe in phase 1 and used throughout:
- Encorafenib 300 mg orally once daily
- Cetuximab 500 mg/m² intravenously every 14 days
- Nivolumab 480 mg intravenously every 28 days
Radiologic assessments were performed every 8 weeks using iRECIST criteria. Co-primary endpoints were safety/tolerability and ORR (complete + partial responses). Secondary endpoints included PFS, OS, duration of response, and disease control rate (DCR).
For correlative science, baseline and on-treatment tissue biopsies (where feasible) were analyzed by RNA-seq, and plasma-derived evRNA was sequenced at baseline and on therapy. Gene set variation analysis (GSVA), KEGG and Hallmark pathway analyses, and immune cell deconvolution were used to link clinical outcomes with molecular signatures. An external dataset of dabrafenib + trametinib + spartalizumab in a similar MSS BRAF V600E mCRC population was used for independent validation.
Results
Patient population and safety
A total of 26 participants with MSS BRAFV600E mCRC were treated. The median age was 59 years (range 32–85); 62% were female. Most patients (62%, n=16) had received just one prior line of systemic therapy, and 58% had right-sided primary tumors. Common metastatic sites included liver (58%), peritoneum (58%), lymph nodes (38%), and lung (35%).
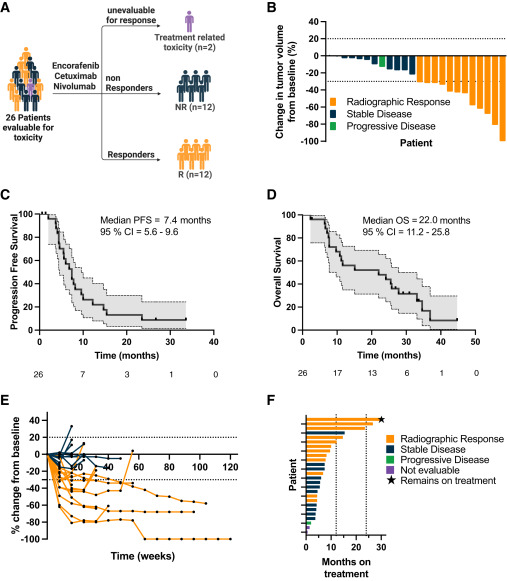
In the dose-finding cohort, no DLTs were observed among the first six patients, so dose level 1 was used across the study. In the full treated cohort, no DLTs and no grade 5 treatment-related adverse events occurred. Grade 3–4 toxicities were infrequent: asymptomatic elevated lipase in 12% and elevated amylase in 8% were the most common high-grade events and were attributed to nivolumab. Single cases of grade 3 colitis, maculopapular rash, leukocytosis, and grade 4 myositis/myocarditis were observed. Two patients discontinued during cycle 1 due to toxicity or infusion reaction and were not evaluable for efficacy.
Across all grades, common adverse events (>20%) included headache (58%), nausea (42%), arthralgia (39%), anemia (31%), acneiform rash (31%), pruritus (27%), and infusion reactions (23%). Six patients developed encorafenib-related keratoacanthomas or squamous cell skin cancers, all managed by local ablation or excision.
Efficacy
Among 24 evaluable patients, there was 1 complete response and 11 partial responses, yielding an ORR of 50% (95% CI, 29–71) and a confirmed ORR of 42% (95% CI, 22–64). In the intention-to-treat population of all 26 treated patients, the ORR was 46% (95% CI, 27–67). The disease control rate (CR + PR + SD) was 96% (95% CI, 79–100); only one patient had primary progression at first restaging. The median duration of response was 7.7 months (95% CI, 4.5–not estimable).
With a median follow-up of 18.1 months, the median PFS was 7.4 months (95% CI, 5.6–9.6), and the median OS was 22.0 months (95% CI, 11.2–25.8). Thirteen patients remained on study at ≥6 months, and two patients (8%) had exceptionally durable benefit, remaining on therapy for about 26.7 and 33.6+ months, respectively.
Response was not associated with sex, but left-sided primaries showed higher response probability than right-sided tumors (58% vs 42%; odds ratio 0.24, p=0.04). Responders had markedly better outcomes than non-responders: median PFS 9.6 vs 5.0 months (HR 0.28, p=0.001) and median OS 30.5 vs 8.7 months (HR 0.31, p=0.003).
Key Findings
Compared with historical data for encorafenib + cetuximab (ORR ~20%, median PFS 4.2 months, median OS 9.1–9.4 months), the triple combination of encorafenib, cetuximab and nivolumab achieved:
- ORR 50% among evaluable patients and 46% in the ITT population
- Median PFS 7.4 months
- Median OS 22.0 months
- Disease control rate 96%
These results more than double both PFS and OS versus prior BRAF+EGFR combinations in this poor-prognosis group.
The regimen was well tolerated without DLTs at full doses, and grade 3–4 toxicity was manageable. Immune-related adverse events such as myositis/myocarditis and colitis were rare but clinically significant and require careful monitoring. Dermatologic toxicities related to encorafenib were expected and manageable.
Bulk RNA-seq of pretreatment biopsies showed that responders were enriched for:
- p38 MAPK signaling
- mmune activation pathways, including TNF, TCR, DAP12, and inflammatory response signatures
- Higher activity of natural killer cells, B cells, T cells, neutrophils, and macrophages
By contrast, non-responders showed:
- Enrichment of complement and coagulation cascade signatures
- Higher expression of immune-suppressive genes (e.g., ARG1, IGF1, NR4A1)
- Stronger activation of classical RAF pathway signaling and multiple metabolic pathways (amino acid, lipid, bile acid, xenobiotic and drug metabolism)
- Complement activation emerged as a negative predictor of response and was mutually exclusive with immune-inflamed signatures.
evRNA profiling from plasma mirrored tissue-based signatures. At baseline, responders’ evRNA samples showed immune activation, while non-responders showed metabolic enrichment. On treatment, responders displayed:
- Decreases in MAPK pathway scores
- Increases in interferon-γ response signatures and cytotoxic T-cell pathways
These dynamic evRNA changes correlated with duration of benefit; the two patients with >2-year responses had the largest on-treatment rise in interferon-γ scores. An external trial of dabrafenib, trametinib, and spartalizumab in MSS BRAF V600E mCRC showed similar patterns, supporting the generalizability of the identified signatures.
Key Takeaway Messages
- The triplet of encorafenib + cetuximab + nivolumab is clinically active and tolerable in MSS BRAF V600E metastatic colorectal cancer, with ORR ~50% and median OS 22 months.
- Benefit appears greater than historical outcomes with BRAF + EGFR inhibition alone, supporting the addition of PD-1 blockade in this molecular subgroup.
- Baseline immune-inflamed tumors with p38 MAPK and interferon-γ–related signatures are more likely to respond, whereas complement and metabolic pathway activation mark resistance.
- Plasma evRNA provides a promising non-invasive tool to capture these signatures and track on-treatment changes, potentially guiding patient selection and early response assessment.
A randomized phase 2 trial (SWOG 2107) is ongoing to confirm whether adding nivolumab to encorafenib + cetuximab should become a new standard for previously treated MSS BRAF V600E mCRC.
Conclusion
This phase 1/2 study shows that encorafenib, cetuximab, and nivolumab can achieve high response rates, durable disease control, and a median OS of nearly two years in MSS BRAF V600E metastatic colorectal cancer, a population with historically poor outcomes. The regimen was tolerable at full doses, with manageable immune-related and dermatologic toxicities.
Correlative tissue and evRNA data suggest that benefit is driven by an inflamed, p38-activated tumor microenvironment with treatment-associated MAPK suppression and interferon-γ activation, whereas non-responders exhibit complement activation and metabolic programs linked to immune suppression and alternative MAPK re-activation.
If validated in the randomized SWOG 2107 trial, this combination could reshape the standard of care for MSS BRAF V600E mCRC and support evRNA-based transcriptional profiling as a feasible biomarker strategy for patient selection and response monitoring.
You can read the full article here.


