Patients with newly diagnosed advanced ovarian cancer often benefit from platinum-based chemotherapy and bevacizumab, while PARP inhibitors provide significant benefit particularly in BRCA-mutated and HRD-positive tumors. However, optimal strategies for BRCA-wild-type (non-tBRCAm) populations remain unclear. DUO-O explored whether adding durvalumab (anti-PD-L1) to chemotherapy/bevacizumab, followed by maintenance durvalumab + bevacizumab with or without olaparib, could improve outcomes.
Study Design
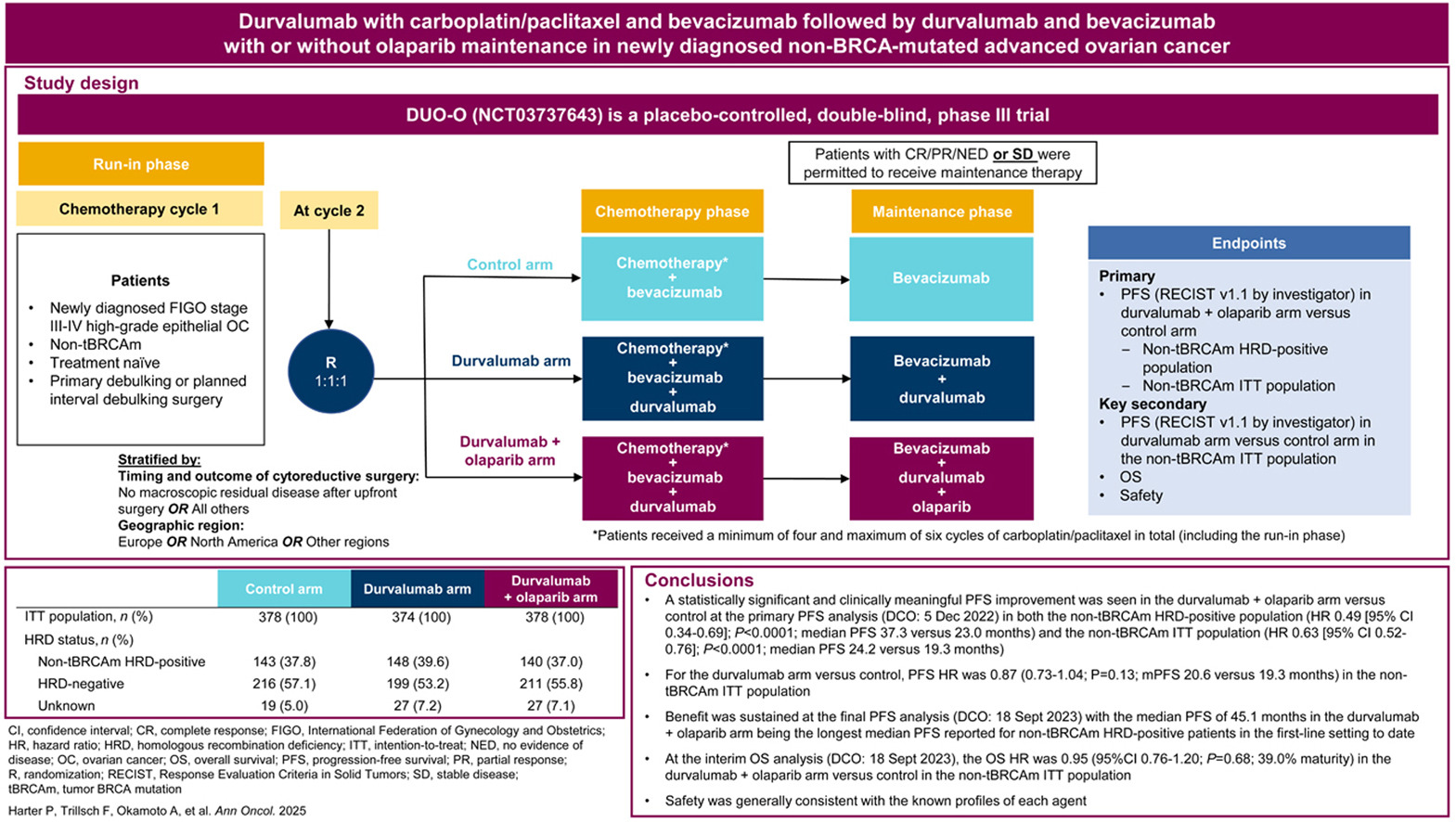
The DUO-O trial (NCT03737643) was a phase III, international, randomized, double-blind, placebo-controlled study evaluating whether adding durvalumab—and in one arm also olaparib—to standard chemotherapy and bevacizumab could improve outcomes for patients with newly diagnosed advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer.
A total of 1,130 patients with FIGO stage III–IV disease and no BRCA mutation were enrolled. Randomization occurred at cycle 2 into three groups in a 1:1:1 ratio:
- Control arm: carboplatin + paclitaxel with bevacizumab, followed by bevacizumab maintenance.
- Durvalumab arm: chemotherapy + bevacizumab + durvalumab during induction, followed by maintenance durvalumab + bevacizumab.
- Durvalumab + olaparib arm: same induction as arm 2, followed by maintenance durvalumab + bevacizumab + olaparib.
Treatment Regimen
- Carboplatin AUC 5–6 + Paclitaxel 175 mg/m² q3w for six cycles
- Bevacizumab 15 mg/kg q3w during induction and throughout maintenance
- Durvalumab 1120 mg q3w in the durvalumab-containing arms
- Olaparib 300 mg twice daily only in the maintenance phase of arm 3
- Interval debulking surgery was permitted after cycle 3
- Treatment continued until disease progression or completion of planned therapy
Endpoints
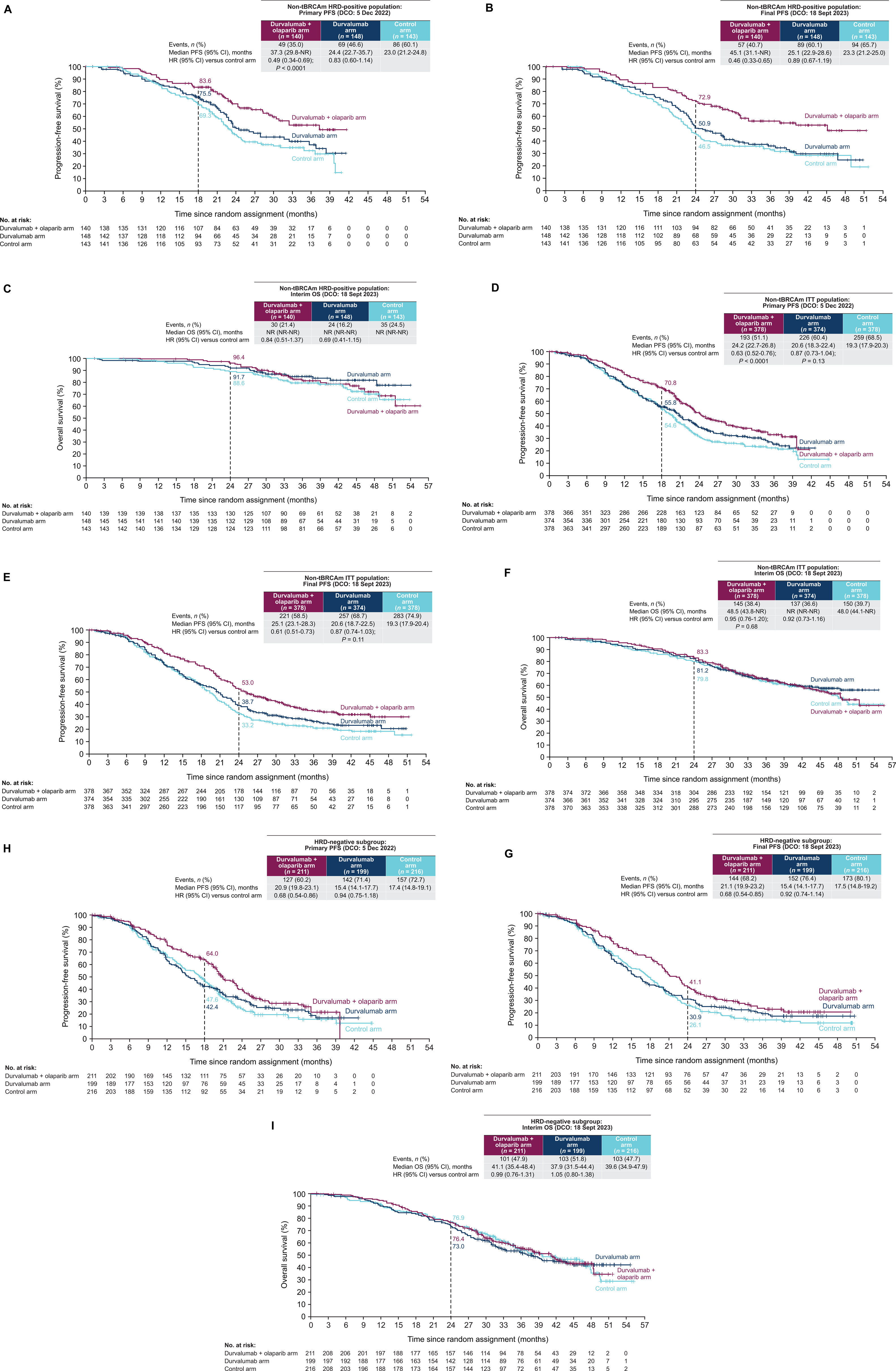
- Primary endpoint: progression-free survival (PFS) first assessed in non-tBRCAm HRD-positive patients, then in the overall intention-to-treat (ITT) population
- Secondary endpoints: overall survival (OS), PFS2, quality of life (QoL), and safety outcomes
Efficacy Overview
The DUO-O trial demonstrated a meaningful improvement in progression-free survival, with the greatest benefit observed in patients receiving durvalumab combined with olaparib. Interim and final analyses were consistent.
In the HRD-positive population, the durvalumab + olaparib regimen reduced the risk of progression by 51% compared with standard therapy (HR 0.49, P<0.0001). Median PFS improved substantially, reaching 37.3–45.1 months, versus 23.0–23.3 months with control treatment.
When assessed across the entire non-BRCA-mutated ITT population, the combination still showed a statistically significant benefit (HR 0.63, P<0.0001), with median PFS extending from 19.3 months in the control arm to 24.2–25.1 months. However, durvalumab alone did not significantly prolong PFS, demonstrating a median of 20.6 months vs 19.3 monthswith control (HR 0.87, not statistically significant).
Overall, the data indicate that the greatest efficacy emerges when durvalumab is paired with olaparib, particularly in HRD-positive disease, though benefit was seen across HRD-negative and other biomarker subgroups as well.
Overall Survival (Interim Results)
Interim overall survival analysis has not yet demonstrated a statistically significant benefit in the intent-to-treat population. The comparison of durvalumab + olaparib versus standard therapy showed an HR of 0.95, indicating no clear OS advantage at this time. However, early signals suggest a possible trend toward improved survival in HRD-positive patients, although the data remain immature. Continued follow-up is ongoing to determine whether this trend will translate into a definitive OS benefit with longer observation.
Safety
Safety reflected known profiles of each agent.
- Grade ≥3 AEs
- Control: 62%
- Durva: 66%
- Durva+Ola: 72% (higher due to anemia & neutropenia from olaparib)
Common AEs
- Nausea, anemia, neutropenia, fatigue, hypertension, GI effects
- Immune-mediated AEs more frequent in durvalumab arms
- MDS/AML very rare
- PRCA/AIHA occurred only in Durva+Ola (<1%), all resolved after discontinuation
- QoL remained similar across arms, no major deterioration observed.
Clinical Takeaway
Durvalumab + bevacizumab + olaparib is an effective first-line option for non-BRCA-mutated advanced ovarian cancer, particularly when HRD-positive. Its integration into evolving treatment algorithms will likely depend on long-term OS data and comparison to other PARPi-immunotherapy combinations.
You can read all article here