DESTINY-Breast11 l presented by Prof. Nadia Harbeck at the ESMO Congress 2025, evaluated trastuzumab deruxtecan (T-DXd) alone or followed by paclitaxel plus trastuzumab and pertuzumab (T-DXd-THP) versus dose-dense doxorubicin plus cyclophosphamide followed by THP (ddAC-THP) as neoadjuvant therapy for high-risk HER2-positive early breast cancer (eBC). The study aimed to determine whether the anthracycline-free T-DXd-THP regimen could achieve superior efficacy and improved tolerability compared with the current standard of care.
Background
The current standard neoadjuvant therapy for HER2-positive early breast cancer combines trastuzumab (H) and pertuzumab (P) with multi-agent chemotherapy. However, anthracycline-containing regimens such as ddAC-THP carry a risk of cardiotoxicity and other adverse effects.
Trastuzumab deruxtecan (T-DXd), a potent HER2-directed antibody–drug conjugate, has demonstrated high antitumor activity in the metastatic setting. This trial explored its potential as a front-line neoadjuvant option capable of replacing anthracyclines while maintaining or improving treatment efficacy.
Methods
Adults with previously untreated, high-risk HER2-positive eBC (≥T3 or node-positive [N1–3], including inflammatory disease) were randomized 1:1:1 to:
- T-DXd-THP: T-DXd 5.4 mg/kg every 3 weeks × 4 cycles → THP × 4 cycles (n=321)
- ddAC-THP: Doxorubicin + cyclophosphamide every 2 weeks × 4 cycles → THP × 4 cycles (n=320)
- T-DXd alone: 5.4 mg/kg every 3 weeks × 8 cycles (n=286)
The primary endpoint was pathologic complete response (pCR; ypT0/is ypN0).Secondary endpoints included event-free survival (EFS), residual cancer burden (RCB), and safety.
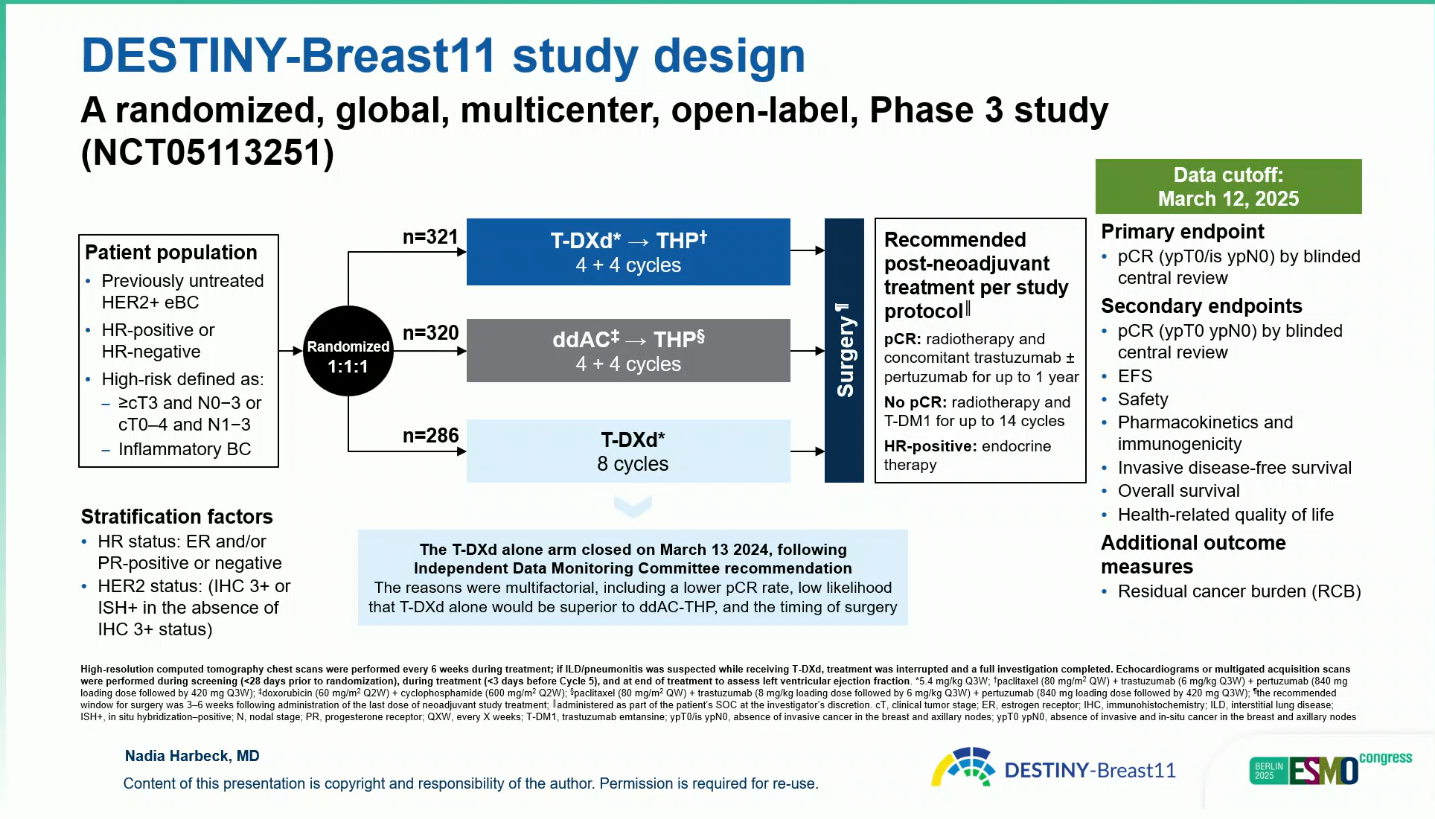
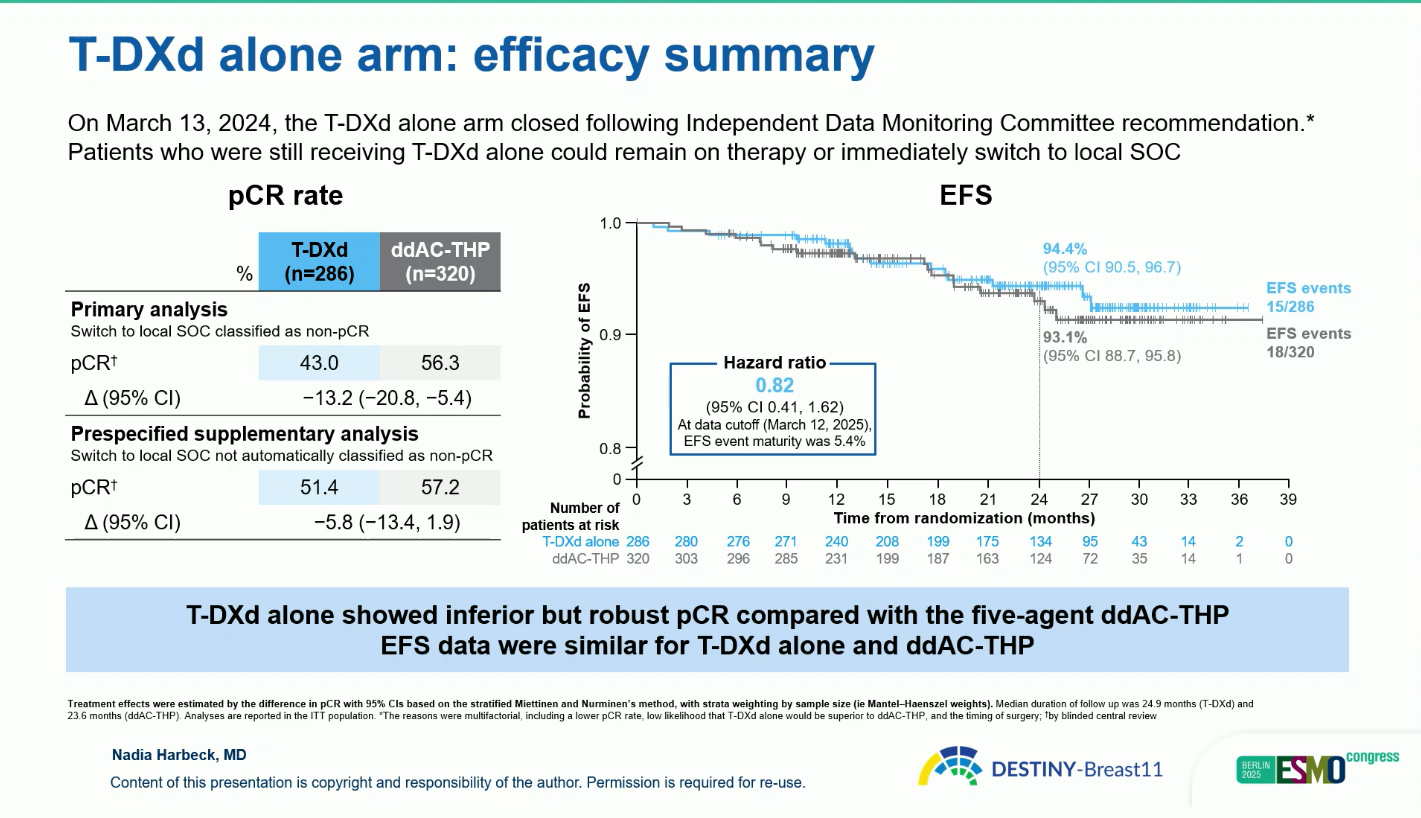
Enrollment in the T-DXd monotherapy arm was closed early (March 2024) following IDMC recommendation due to a lower pCR rate and unlikely superiority versus ddAC-THP.
Results
As of the March 12, 2025 data cut-off, participants were randomized 1:1:1 to T-DXd-THP (n = 321), ddAC-THP (n = 320), or T-DXd alone (n = 286).
- The pCR rate was 67.3 percent with T-DXd-THP compared with 56.3 percent with ddAC-THP, representing an absolute difference of 11.2 percentage points (95 percent confidence interval 4.0 to 18.3; p = 0.003)
.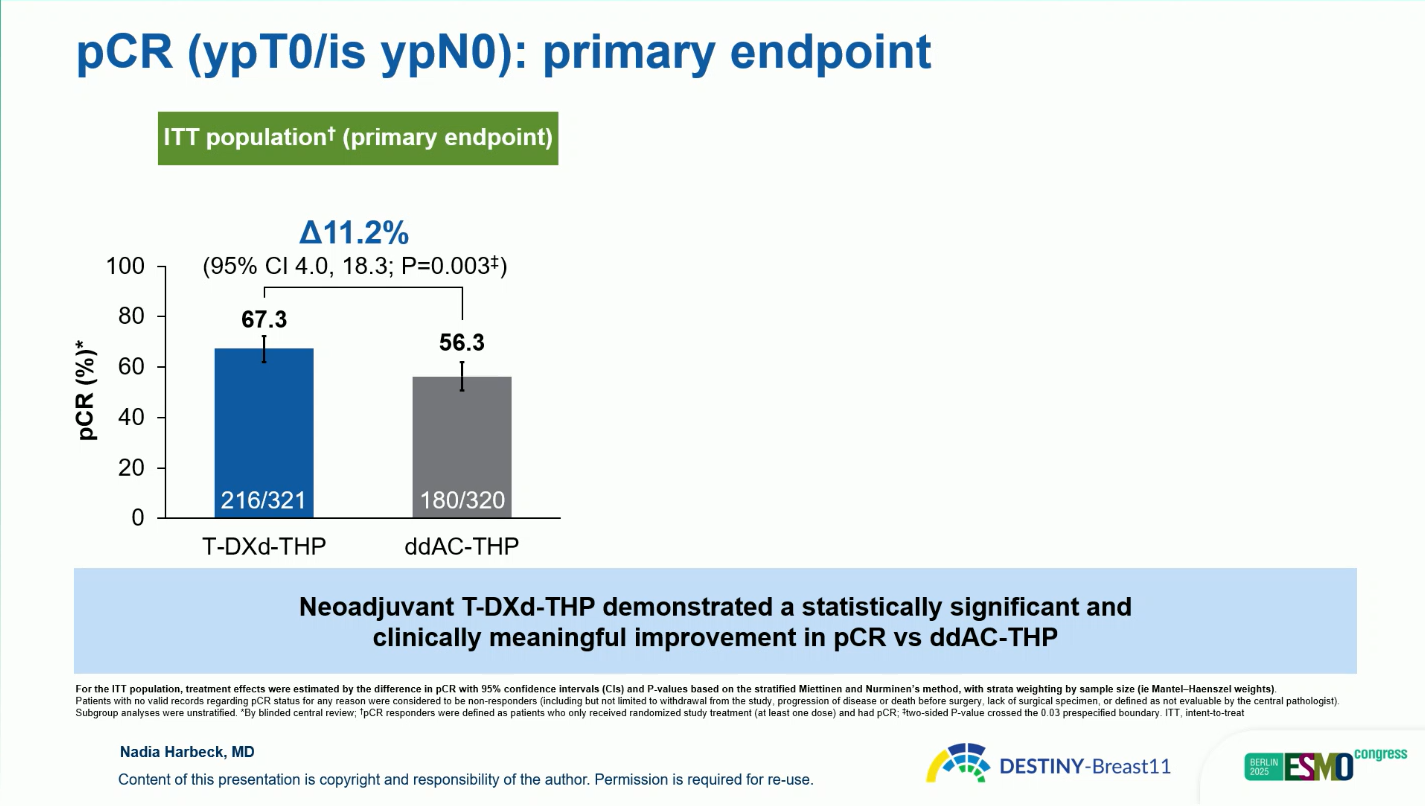
Improvement was consistent across subgroups: for hormone-receptor-positive disease, pCR was 61.4 versus 52.3 percent, and for hormone-receptor-negative disease, 83.1 versus 67.1 percent, respectively.
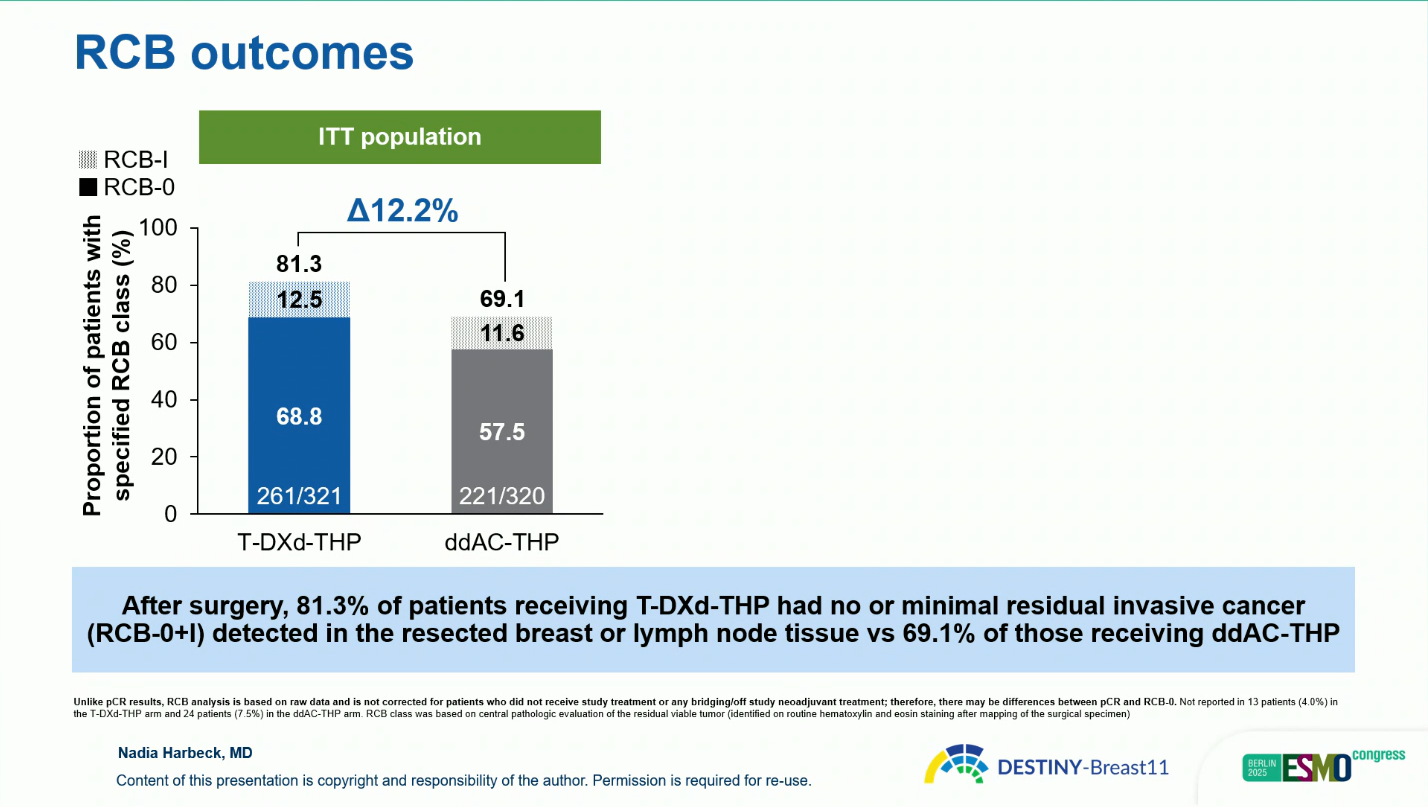
- Analysis of residual cancer burden showed a higher rate of minimal or no residual disease (RCB 0 or I) with T-DXd-THP—81.3 percent compared with 69.1 percent for ddAC-THP—confirming a deeper pathologic response.
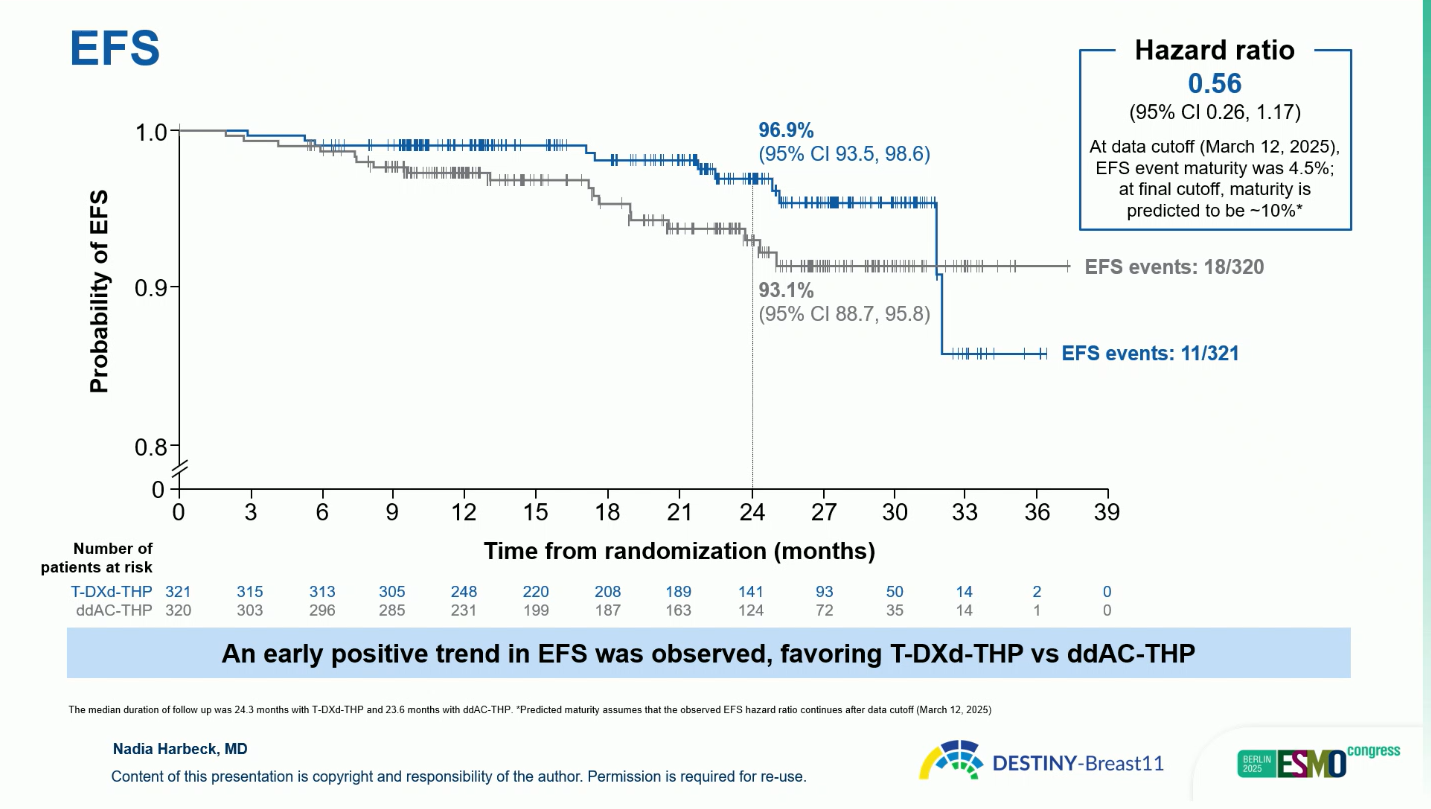
- At the time of interim follow-up, an early favorable EFS trend was observed with T-DXd-THP, with a hazard ratio of 0.56 (95 percent CI 0.26 to 1.17). The estimated two-year EFS rate was 96.9 percent for T-DXd-THP and 93.1 percent for ddAC-THP. Although EFS data remain immature (4.5 percent maturity), the early separation of curves suggests a potential durable benefit.
Safety
The T-DXd-THP regimen was associated with fewer serious and high-grade adverse events than ddAC-THP. Grade 3 or higher events occurred in 37.5 percent of patients receiving T-DXd-THP versus 55.8 percent with ddAC-THP, and serious adverse events in 10.2 versus 20.2 percent, respectively. Interstitial lung disease or pneumonitis occurred in 4.9 percent and 5.1 percent of patients in the two arms, respectively, with the majority being grade 1 or 2 and manageable with appropriate intervention.
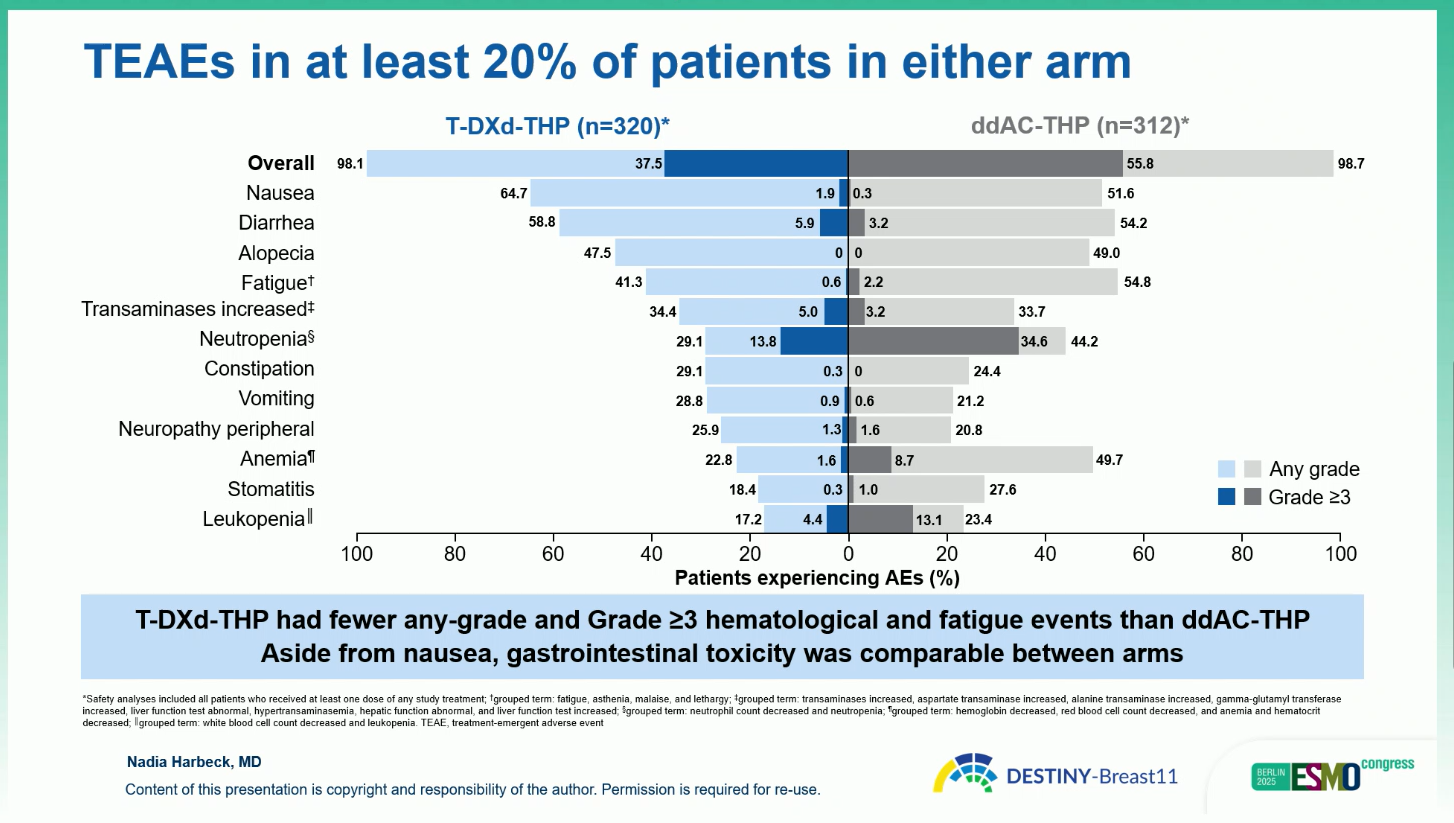
Rates of left ventricular dysfunction were notably lower in the T-DXd-THP arm (1.9 percent versus 9.0 percent). No adverse event prevented surgery in any arm, and overall treatment discontinuation due to toxicity was less frequent with T-DXd-THP than with ddAC-THP. Gastrointestinal events, such as nausea and diarrhea, were common but manageable and comparable between arms.
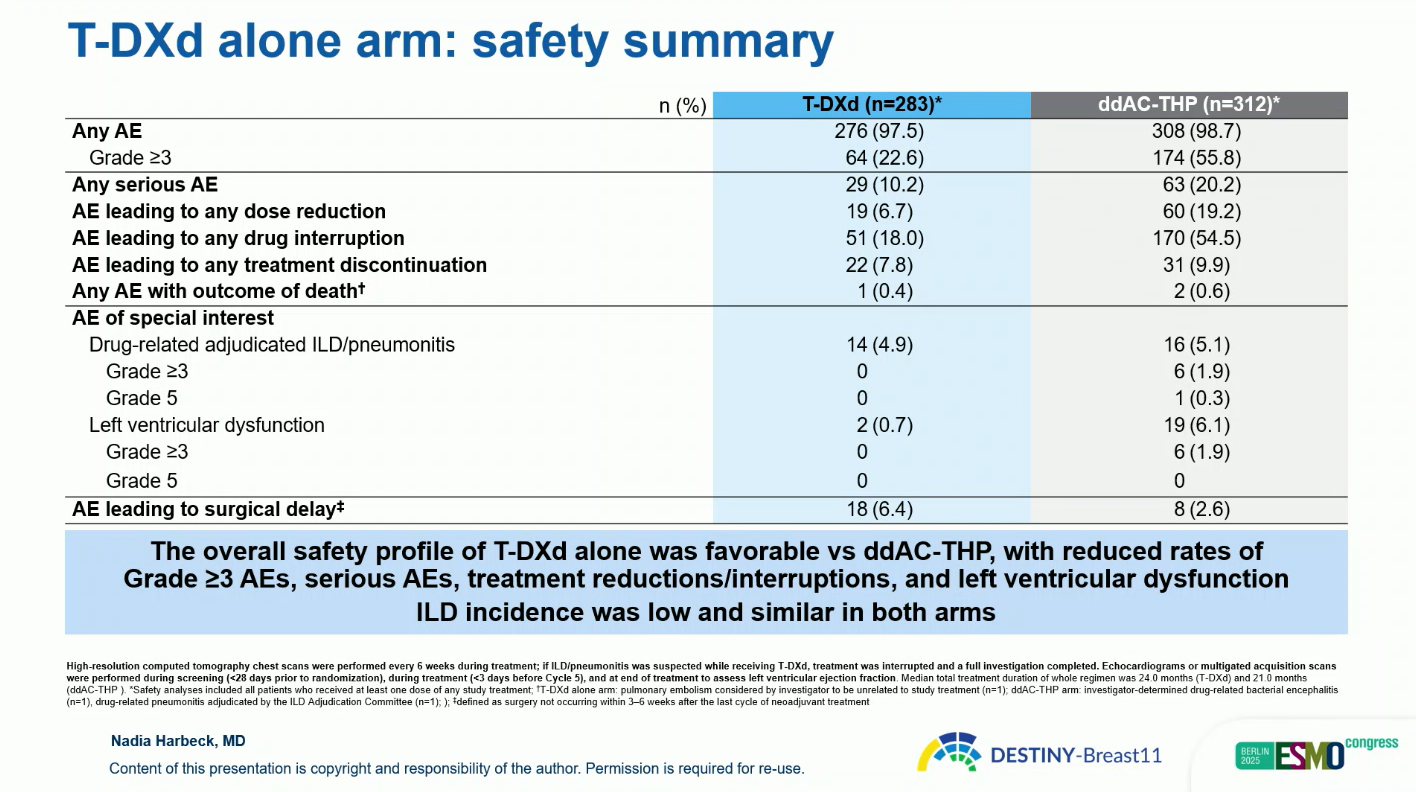
The monotherapy arm of T-DXd showed a pCR rate of 43 percent when switchers were classified as non-responders, or 51.4 percent in a sensitivity analysis. Although inferior to combination therapy, it was associated with fewer grade 3 and serious events and low ILD incidence (4.9 percent, mostly grade 1–2, none fatal).
Conclusions
The T-DXd-THP regimen achieved a statistically significant and clinically meaningful improvement in pCR compared with ddAC-THP, alongside an early favorable EFS signal and improved safety profile. These findings establish T-DXd-THP as a potential new anthracycline-free neoadjuvant standard of care for patients with high-risk HER2-positive early breast cancer, offering enhanced efficacy and reduced toxicity.
You can read the full abstract here.


