Datopotamab-Deruxtecan (Datroway), a TROP2-directed antibody-drug conjugate jointly developed by AstraZeneca and Daiichi Sankyo, has achieved a historic milestone in the treatment of triple-negative breast cancer (TNBC). In the Phase III TROPION-Breast02 trial, Datroway became the first and only therapy to show a statistically significant and clinically meaningful improvement in overall survival (OS) compared to chemotherapy as first-line treatment for patients with locally recurrent inoperable or metastatic TNBC who are not eligible for immunotherapy.
The results also demonstrated a substantial benefit in progression-free survival (PFS), marking a major advance for this aggressive subtype of breast cancer, where therapeutic options remain limited and outcomes poor.
Crash Course on TNBC
Triple-negative breast cancer (TNBC) accounts for about 15% of all breast cancer cases, translating to roughly 345,000 diagnoses globally each year. It is more common among younger and premenopausal women and shows higher prevalence in Black and Hispanic populations.
Unlike other subtypes, TNBC lacks expression of estrogen receptor (ER), progesterone receptor (PR), and HER2, making it difficult to treat and associated with poor outcomes. The median overall survival (OS) in metastatic TNBC remains only 12–18 months, with approximately 14% of patients alive at five years.
For about 70% of patients whose tumors do not express PD-L1—or who cannot receive immunotherapy because of prior exposure, comorbidities, or accessibility issues—chemotherapy remains the first-line standard of care. This group has long faced an urgent need for new, effective options.
What drug is Datopotamab-Deruxtecan and how does it work?
Datroway (datopotamab deruxtecan) is a TROP2-directed antibody-drug conjugate (ADC) that combines targeted delivery with potent cytotoxic activity. It is composed of a humanized anti-TROP2 IgG1 monoclonal antibody linked to a topoisomerase I inhibitor payload (DXd, an exatecan derivative) via a cleavable tetrapeptide-based linker.
Once the antibody binds to the TROP2 receptor, which is highly expressed in many solid tumors including triple-negative breast cancer, the ADC is internalized into the cancer cell. Inside the cell, the linker is cleaved, releasing the DXd payload that inhibits topoisomerase I, leading to DNA damage, cell cycle arrest, and apoptosis. This targeted mechanism allows for selective killing of tumor cells while minimizing systemic toxicity, representing a major advancement in precision oncology for hard-to-treat cancers like TNBC.
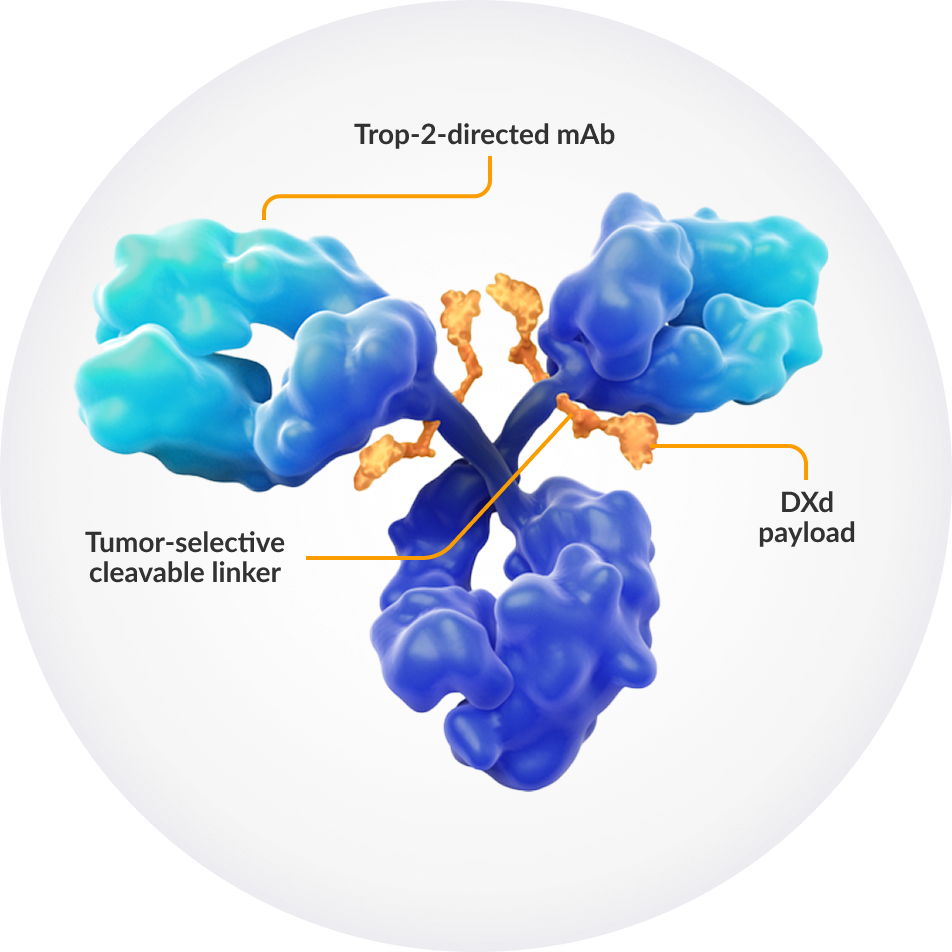
Source: https://datrowayhcp.com/mechanism-of-action
About TROPION-Breast02 trial
The TROPION-Breast02 trial is a global, multicenter, randomized, open-label, Phase III study comparing Datroway against investigator’s choice of chemotherapy (paclitaxel, nab-paclitaxel, capecitabine, carboplatin, or eribulin) as first-line therapy for patients with locally recurrent inoperable or metastatic TNBC ineligible for immunotherapy.
The trial enrolled 644 patients across Africa, Asia, Europe, North America, and South America. It included both de novo and recurrent disease, regardless of disease-free interval, and permitted enrollment of patients with poor prognostic factors, such as brain metastases.
The dual primary endpoints were progression-free survival (PFS) assessed by blinded independent central review and overall survival (OS). Secondary endpoints included PFS by investigator, objective response rate (ORR), duration of response (DoR), disease control rate (DCR), pharmacokinetics, and safety.
Results
Datroway demonstrated a statistically significant and clinically meaningful improvement in both OS and PFS compared to chemotherapy.
Although detailed numeric data will be presented at an upcoming medical meeting, AstraZeneca and Daiichi Sankyo confirmed that Datroway achieved superiority for both dual primary endpoints—making TROPION-Breast02 the first trial ever to show an OS benefit in first-line metastatic TNBC for patients not eligible for immunotherapy.
The safety profile of Datroway was consistent with previous clinical trials of the drug in breast cancer, with no new safety signals observed.
Key Insights
- Datroway becomes the only therapy to significantly extend OS versus chemotherapy in this patient population.
- Datroway is a TROP2-directed antibody-drug conjugate (ADC) engineered using Daiichi Sankyo’s proprietary DXd technology. It consists of an anti-TROP2 IgG1 monoclonal antibody linked to a topoisomerase I inhibitor payload.
- The results may redefine the first-line treatment paradigm for metastatic TNBC without immunotherapy options, potentially shifting the standard of care.
- Enrollment from five continents ensures strong external validity, highlighting the worldwide applicability of these findings.

Dr. Susan Galbraith, Executive Vice President of Oncology R&D at AstraZeneca, emphasized:
“TROPION-Breast02 is the only trial ever to show an overall survival benefit in first-line treatment of patients with metastatic triple-negative breast cancer for whom immunotherapy is not an option. We expect these results will mark an inflection point in treatment.”

Dr. Ken Takeshita, Global Head of R&D at Daiichi Sankyo, added:
“Datroway is the first ADC and only therapy to significantly improve overall survival compared with chemotherapy in this difficult-to-treat setting. These results strengthen our confidence in the broader Datroway program.”
The Datopotamab-Deruxtecan Development Program
Datroway (datopotamab deruxtecan; datopotamab deruxtecan-dlnk in the U.S.) is already approved in over 35 countries for unresectable or metastatic HR-positive, HER2-negative breast cancer after prior endocrine-based therapy and chemotherapy, based on TROPION-Breast01 results.
A comprehensive global program is evaluating Datroway in more than 20 trials across tumor types, including NSCLC, TNBC, and urothelial cancer. In TNBC, additional Phase III trials include:
- TROPION-Breast03: Datroway ± Imfinzi in Stage I-III TNBC with residual disease after neoadjuvant therapy.
- TROPION-Breast04: Neoadjuvant Datroway + Imfinzi in Stage II-III TNBC or HR-low, HER2-low/negative breast cancer.
- TROPION-Breast05: Datroway ± Imfinzi in PD-L1-positive metastatic TNBC.
Conclusion
The results from the TROPION-Breast02 trial represent a pivotal advancement in the treatment landscape of metastatic triple-negative breast cancer. For the first time, a therapy—Datroway (datopotamab deruxtecan)—has demonstrated a significant overall survival benefit over standard chemotherapy in patients who are not candidates for immunotherapy.
This achievement not only underscores the therapeutic potential of TROP2-directed antibody-drug conjugates but also offers new hope for a patient population historically associated with poor prognosis and limited treatment options. As AstraZeneca and Daiichi Sankyo prepare to present the full data and engage with global regulatory authorities, Datroway is positioned to reshape the first-line standard of care in metastatic TNBC, setting a new benchmark for innovation in breast cancer therapy.
You can read the press release here.


