The FDA has granted priority review to a supplemental biologics license application for datopotamab deruxtecan(Dato-DXd) as first-line treatment for adult patients with unresectable or metastatic triple-negative breast cancer (TNBC) who are not eligible for PD-1– or PD-L1–directed immunotherapy.
The Prescription Drug User Fee Act (PDUFA) target action date is expected in the second quarter of 2026.
Regulatory Submission Supported by TROPION-Breast02
The application is based on results from the global, open-label, phase 3 TROPION-Breast02 trial, presented at the 2025 ESMO Congress.
The study enrolled patients with locally recurrent unresectable or metastatic TNBC who had not received prior systemic therapy in the advanced setting and were ineligible for immunotherapy.
Patients were randomized 1:1 to receive Dato-DXd or investigator’s choice of chemotherapy, including paclitaxel, nab-paclitaxel, capecitabine, eribulin, or carboplatin.
Overall Survival Benefit in a High-Risk Population
Treatment with Dato-DXd resulted in a 21% reduction in the risk of death compared with chemotherapy (HR, 0.79; 95% CI, 0.64–0.98; P = .0291).
Median overall survival was 23.7 months with Dato-DXd versus 18.7 months with chemotherapy, representing a clinically meaningful improvement in a population with historically poor outcomes.
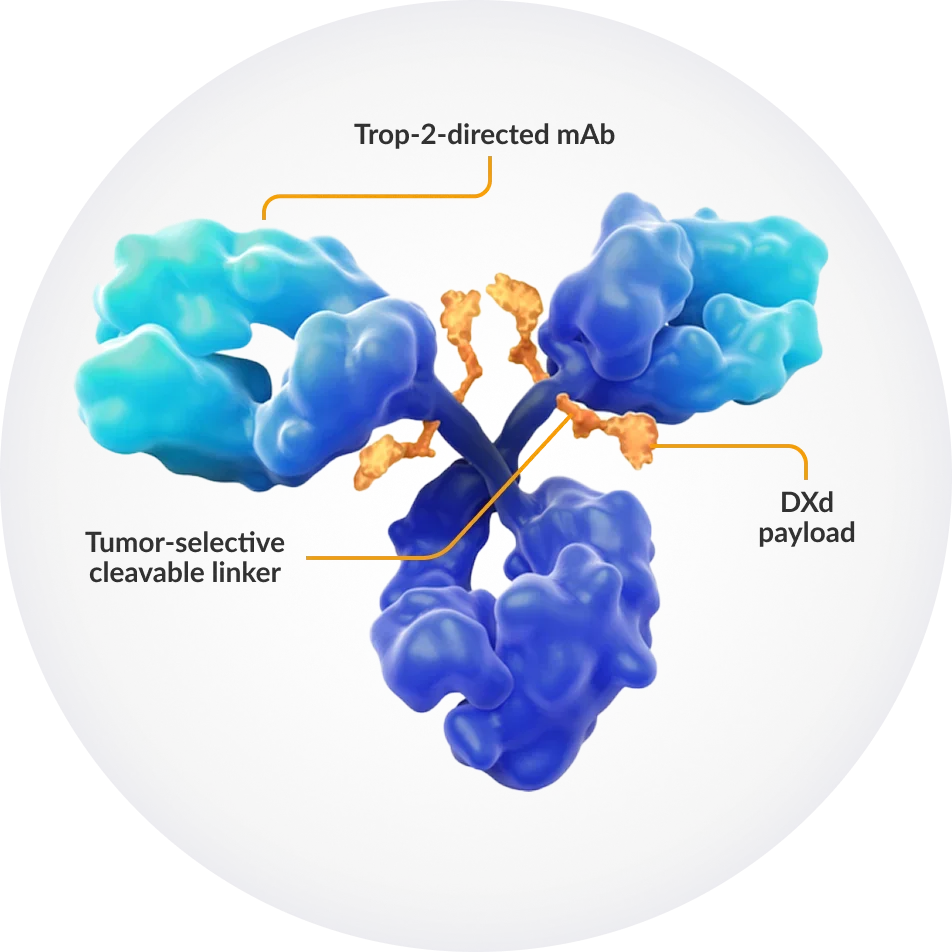 Source: https://datrowayhcp.com/mechanism-of-action
Source: https://datrowayhcp.com/mechanism-of-action
Significant Improvement in Progression-Free Survival
Dato-DXd also demonstrated a substantial benefit in disease control, with a 43% reduction in the risk of disease progression or death (HR, 0.57; 95% CI, 0.47–0.69; P < .0001).
Median progression-free survival was 10.8 months in the Dato-DXd arm compared with 5.6 months in the chemotherapy arm, nearly doubling time without progression.
Safety Profile
The safety profile of Dato-DXd was consistent with prior breast cancer studies.
Among patients receiving Dato-DXd, most experienced treatment-related adverse events, with 33% developing grade 3 or higher toxicities.
The most common treatment-related adverse events included stomatitis, nausea, dry eye, constipation, vomiting, and decreased appetite. No new safety signals were reported in this frontline setting.
Clinical Implications
Patients with metastatic TNBC who are ineligible for immunotherapy represent a population with limited frontline options and aggressive disease biology.
Dato-DXd is the first agent to demonstrate a statistically significant overall survival advantage over chemotherapyin this specific first-line setting, positioning it as a potential new standard of care pending regulatory approval.

You Can Read About Press Release: Datopotamab-Deruxtecan Demonstrates Survival Benefit in First-Line Metastatic Triple-Negative Breast Cancer


