Gynecologic clear cell carcinomas (CCCs) of the ovary, endometrium, and cervix are rare, chemotherapy-resistant, and clinically aggressive. Single-agent PD-1/PD-L1 blockade has generally produced modest response rates, creating a rationale to test dual checkpoint inhibition (CTLA-4 + PD-1) as a way to deepen and prolong immune responses—especially in biologically distinct tumors such as ovarian CCC (often enriched for ARID1A/PI3K-pathway alterations and endometriosis-associated inflammation).
Study Design and Treatment
DART (SWOG S1609) is a multicenter, rare-tumor phase II basket trial. This dedicated cohort enrolled 32 evaluable patients with gynecologic CCC:
- Ovarian (n=19), Endometrial (n=8), Cervical (n=5)
- Heavily pretreated: 1–8 prior lines, including 3 with prior PD-1 exposure
Treatment:
- Nivolumab 240 mg IV q2 weeks
- Ipilimumab 1 mg/kg IV q6 weeks
Primary endpoint: RECIST ORR
Key secondary endpoints: iRECIST ORR, PFS, OS, clinical benefit rate (CBR = response + SD ≥6 months), and safety.
Results
In this dedicated DART cohort of 32 patients with gynecologic clear cell carcinoma, dual checkpoint blockade produced low overall response rates, but a clear durability signal in a small subset—most notably in ovarian CCC.
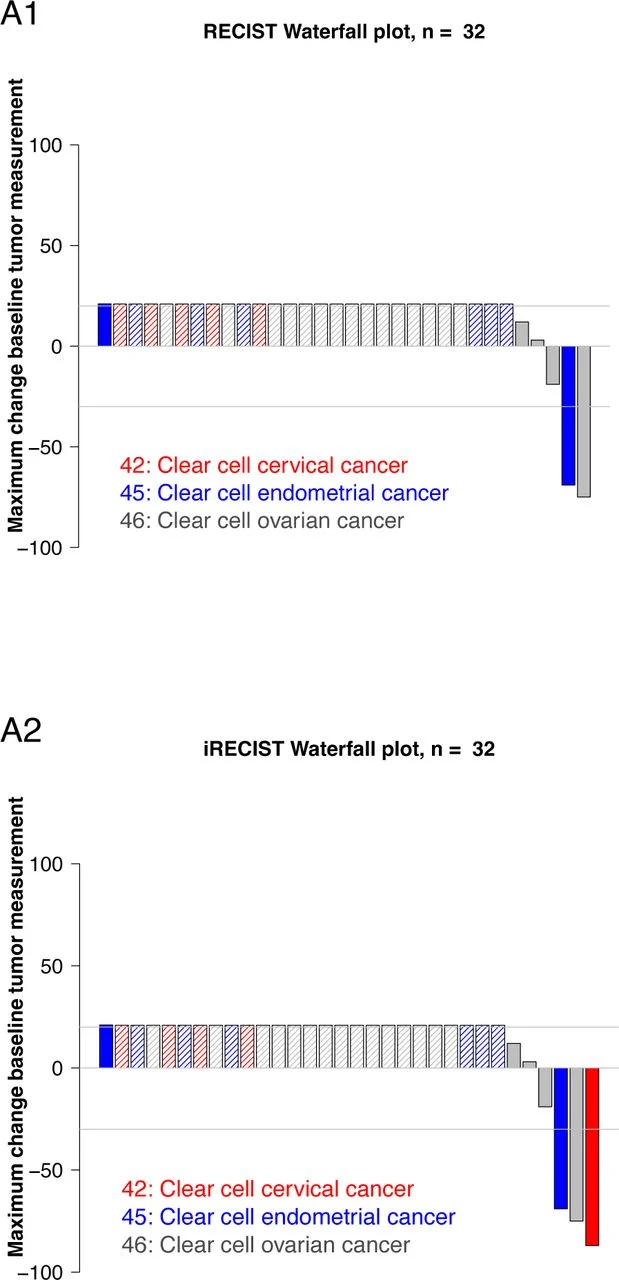
By RECIST v1.1, the overall response rate (ORR) was 9.38% (3/32), consisting of two complete responses (CRs) and one partial response (PR). Importantly, both CRs occurred in ovarian clear cell carcinoma and remained ongoing beyond 3 years, representing the most practice-relevant efficacy signal of the study.
When responses were additionally assessed using immune RECIST (iRECIST), the ORR increased to 12.5% (4/32)because one patient with cervical CCC achieved an immune-confirmed PR lasting 26 months, with an associated overall survival of 32.0 months—again emphasizing that durable benefit can occur even when the average ORR is modest.
Looking beyond response alone, the clinical benefit rate (CBR)—defined as CR/PR or stable disease lasting ≥6 months—was 21.88% (7/32). This indicates that roughly one in five patients achieved meaningful, sustained disease control, largely concentrated in the ovarian and cervical subgroups.
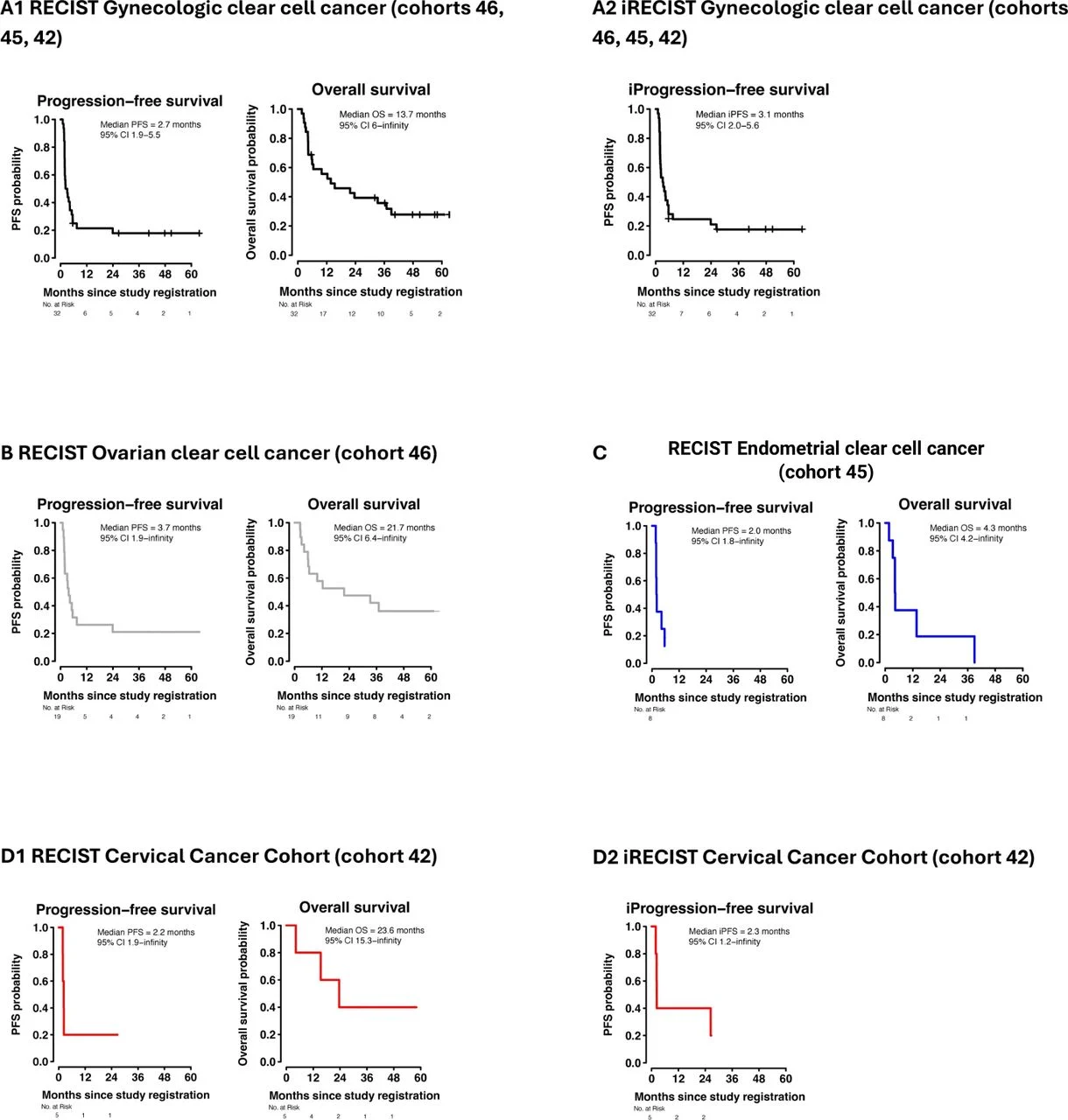
Across the entire cohort, median overall survival (OS) was 21.7 months, but outcomes were heterogeneous. In descriptive, small-number subgroup patterns, ovarian CCC showed the clearest activity (including the deep, multi-year CRs), whereas endometrial CCC demonstrated no clear clinical benefit in this dataset.
Durability signal (the “why this trial is important”)
Even with a low ORR, the key message is durability:
- Two ovarian CCC patients achieved complete remissions lasting ~40–48+ months
- Multiple patients had prolonged disease control (some >3.5–5 years PFS in the CBR group)
Safety
- Grade ≥3 AEs possibly related to treatment: 53% (17/32)
- Discontinued due to toxicity: 22% (7/32)
- No treatment-related deaths
- Common grade 3–4 events highlighted: transaminase elevations, anemia, nausea (immune-toxicity pattern consistent with dual checkpoint therapy).
Insights
Low ORR, but a real “tail” in selected patients
This cohort illustrates a classic dual-checkpoint phenomenon: few responders, but responders can achieve exceptional durability, including multi-year CRs—particularly in ovarian CCC.
Biology likely differs by primary site
The observed activity clustering in ovarian CCC supports the hypothesis that ovarian CCC may be more immunotherapy-permissive than endometrial/cervical CCC, potentially related to differences in genomic drivers and immune microenvironment.
The dosing strategy reduced ipilimumab intensity, but toxicity remained meaningful
Even with lower-frequency ipilimumab (q6 weeks), discontinuation due to toxicity was ~22%, reinforcing that regimen optimization (dose/schedule or combinations with better tolerability) remains an active need.
Why results differ across similar studies (important for interpretation)
When compared with other dual-checkpoint reports in extra-renal/gynecologic CCC, DART’s ORR appears lower—plausibly because this cohort was more heavily pretreated (up to 8 prior lines) and included some prior PD-1 exposure, both of which can reduce apparent ORR even if durability remains possible.
Key Takeaway Messages
- Dual CTLA-4/PD-1 blockade demonstrated clinically meaningful durability in a subset of patients with gynecologic clear cell carcinoma (CCC), despite a modest overall response rate. While RECIST ORR was 9.4%, durable complete responses exceeding three years were observed, underscoring the biological relevance of combination immunotherapy in selected patients.
- Ovarian clear cell carcinoma appears to be the most immunotherapy-responsive subtype within gynecologic CCC. All confirmed complete responses occurred in ovarian CCC, suggesting potential site-specific immunobiologic vulnerability.
- Durability, rather than response frequency, defines the therapeutic signal. The clinical benefit rate of 21.9% and multi-year progression-free intervals in responders highlight that a minority of patients can achieve sustained disease control.
- Activity in endometrial CCC was limited in this cohort, reinforcing biological heterogeneity across gynecologic CCC subtypes. This supports the need for histology- and molecular-informed patient selection strategies.
- Toxicity remains substantial but manageable, consistent with established nivolumab–ipilimumab safety profiles. Treatment discontinuation due to adverse events occurred in 22% of patients, emphasizing the importance of careful patient monitoring and potential dose optimization in future studies.
- Future development should prioritize biomarker-driven selection and translational correlatives to identify the immunologically susceptible subset most likely to benefit from dual checkpoint blockade in this rare and aggressive disease.
Read Full Article Here