Immune checkpoint inhibitors have rapidly expanded across oncology since 2011, yet evidence guiding therapeutic decisions after progression on single-agent PD-1/PD-L1 blockade remains limited, particularly in rare cancers where robust clinical trial data are scarce. The National Cancer Institute/SWOG S1609 (DART) trial provides an unprecedented dataset to evaluate whether responses to dual checkpoint blockade differ between patients previously exposed to anti–PD-1/L1 and those who were immunotherapy-naïve.
This multi-center, prospective, rare-cancer basket trial (NCT02834013) enrolled 729 patients across >50 histologic subtypes at over 1,000 U.S. sites. Participants received nivolumab (240 mg q2w) plus low-dose ipilimumab (1 mg/kg q6w) irrespective of tumor histology. Importantly, prior PD-1/L1 therapy was permitted, enabling direct comparison of response and survival outcomes across the two exposure groups.
Only 35 patients (5%) had prior PD-1/L1 exposure, with this cohort having more prior lines of therapy and higher representation of gastrointestinal and lung primary tumors. Despite these differences, oncologic outcomes were nearly identical between previously treated and untreated patients.
Efficacy Findings
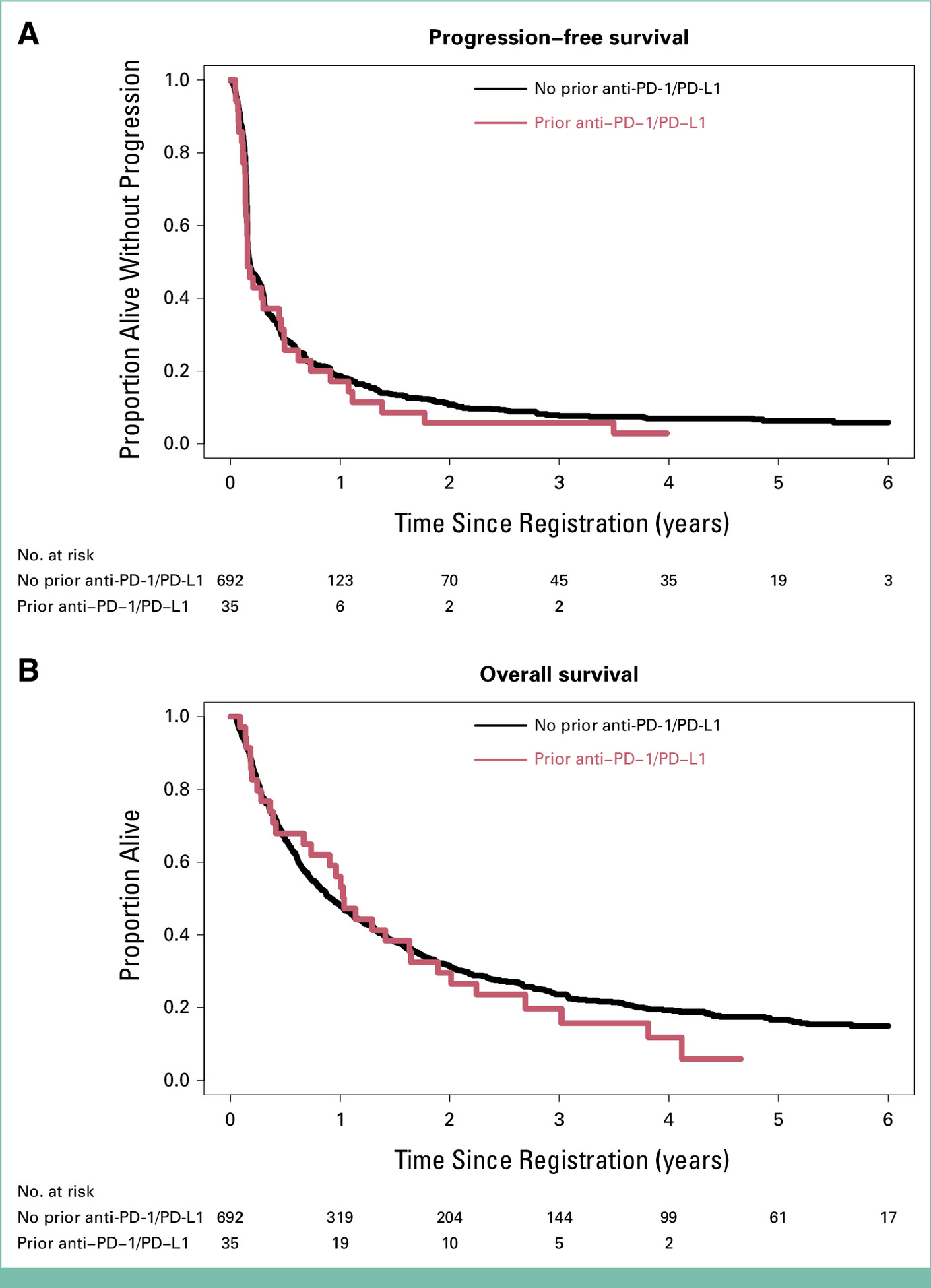
Tumor response was evaluated per RECIST v1.1. The clinical benefit rate (CBR — confirmed CR/PR + SD ≥ 6 months) was 26% in both groups, showing no reduction in benefit among patients previously treated with PD-1/L1 inhibitors. Multivariable Cox regression confirmed no significant difference in progression-free survival or overall survival:
- PFS HR 1.18 (95% CI 0.83–1.68; p = .36)
- OS HR 1.11 (95% CI 0.76–1.63; p = .58)
Kaplan-Meier curves demonstrated nearly overlapping survival patterns, suggesting that prior PD-1/L1 therapy does not diminish response potential to nivolumab + ipilimumab in rare cancers. These results support the continued use of dual checkpoint blockade after prior PD-1 therapy — a clinically relevant finding in treatment-refractory populations.
Safety and Toxicity Profile
Toxicity was common but expected for ipilimumab plus nivolumab, with overall treatment-related adverse events reported in approximately 77% of patients—a rate consistent with established safety data for dual checkpoint blockade. Interestingly, toxicity patterns differed based on previous exposure to PD-1/PD-L1 inhibitors. Patients who had not received prior PD-1/L1 therapy experienced higher rates of immune-related adverse events, whereas those with previous exposure had comparatively fewer toxicities.
- Any immune-related AE: 78% in the PD-1-naïve group vs 54% in previously treated patients (p = .003)
- Grade ≥3 immune-related AE: 38% vs 20%, respectively (p = .031)
Researchers note that this difference likely reflects patient selection, as individuals with significant prior immune-related toxicity would typically be excluded from rechallenge protocols. Even so, the findings indicate that combination nivolumab + ipilimumab is generally tolerable after prior PD-1/L1 therapy, and no unexpected or worsened safety signals emerged in this setting.
Context Within Existing Evidence
Retreatment with ICIs after PD-1 progression has been historically guided by small retrospective series. Reported response rates range 16–29% in melanoma and ≈23% in renal cell carcinoma, aligning closely with this study’s 26% CBR. Mechanistically, activity after PD-1 failure may reflect CTLA-4–mediated immune modulation, PD-L2 upregulation, or reinvigoration of exhausted T-cell pools. The DART trial strengthens these observations in a prospective, histology-agnostic rare-cancer population.
Limitations
The previously treated cohort was small (n=35 across 22 cohorts), limiting subtype-specific interpretation. Details of prior PD-1 therapy — duration, reason for discontinuation, treatment-free interval — were not captured. The study also cannot determine whether up-front combination therapy vs sequential therapy yields superior outcomes, an unanswered question requiring future trial design.
Scientific Implication
The S1609/DART results provide the strongest evidence to date that prior exposure to PD-1/PD-L1 therapy does not negatively impact survival outcomes or clinical benefit with nivolumab + ipilimumab in rare cancers. These data support trial eligibility and clinical use of dual checkpoint blockade following anti-PD-1/L1 monotherapy, filling a major gap in real-world decision-making for rare tumor types.
Clinical Takeaway
- Dual IO (Nivo + Ipi) retains activity after PD-1 therapy.
- CBR, PFS, OS remain comparable whether or not patients previously received anti-PD-1/L1.
- Toxicity is manageable and lower in prior-exposed patients.
- Patients should not be excluded from combination immunotherapy trials due to prior PD-1 use.
You Can Read All Article Here