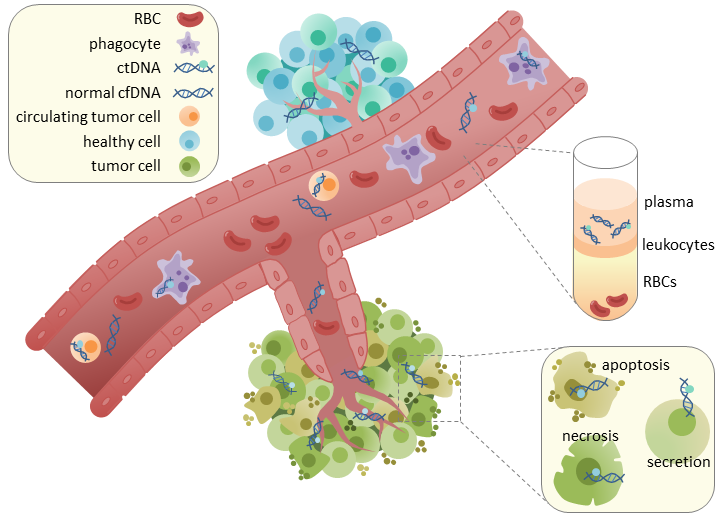
ctDNA (Circulating tumor DNA) has emerged as a transformative biomarker in oncology, offering a minimally invasive method to monitor tumor dynamics and therapeutic responses in real time. As precision medicine continues to shape cancer care, ctDNA has shown particular promise in the neoadjuvant setting, where early identification of responders to immune checkpoint inhibitors (ICIs) could guide treatment decisions and potentially spare patients from unnecessary toxicity.
This evolving biomarker not only reflects the biological behavior of tumors but may also predict outcomes such as pathologic complete response (pCR), a surrogate for long-term survival in many solid tumors. The study under review explores the clinical relevance of ctDNA clearance as a predictive tool for pCR, aiming to refine patient selection and personalize immunotherapy strategies.
Title: Circulating tumor DNA Clearance as a Predictive Biomarker of Pathologic Complete Response in Patients with Solid Tumors Treated with Neoadjuvant Immune-Checkpoint Inhibitors: a Systematic Review and Meta-Analysis
Authors: C. Valenza, E.F. Saldanha, Y. Gong, P. De Placido, D. Gritsch, H. Ortiz, D. Trapani, F. Conforti, C. Cremolini, S. Peters, J. Mateo, V. Subbiah, H.A. Parsons, A.H. Partridge, G. Curigliano
Published in Annald of Oncology , April 2025
Background
Neoadjuvant immune checkpoint inhibitors (ICIs) have revolutionized the treatment landscape for various solid tumors. Achieving a pathologic complete response (pCR) post-treatment is indicative of favorable long-term outcomes. Identifying biomarkers that can predict pCR preoperatively would significantly aid in tailoring treatment strategies. Circulating tumor DNA (ctDNA), fragments of DNA shed by tumors into the bloodstream, has emerged as a potential non-invasive biomarker. This study aims to evaluate the predictive value of ctDNA clearance for pCR in patients undergoing neoadjuvant ICI therapy.
Study Design and Methods
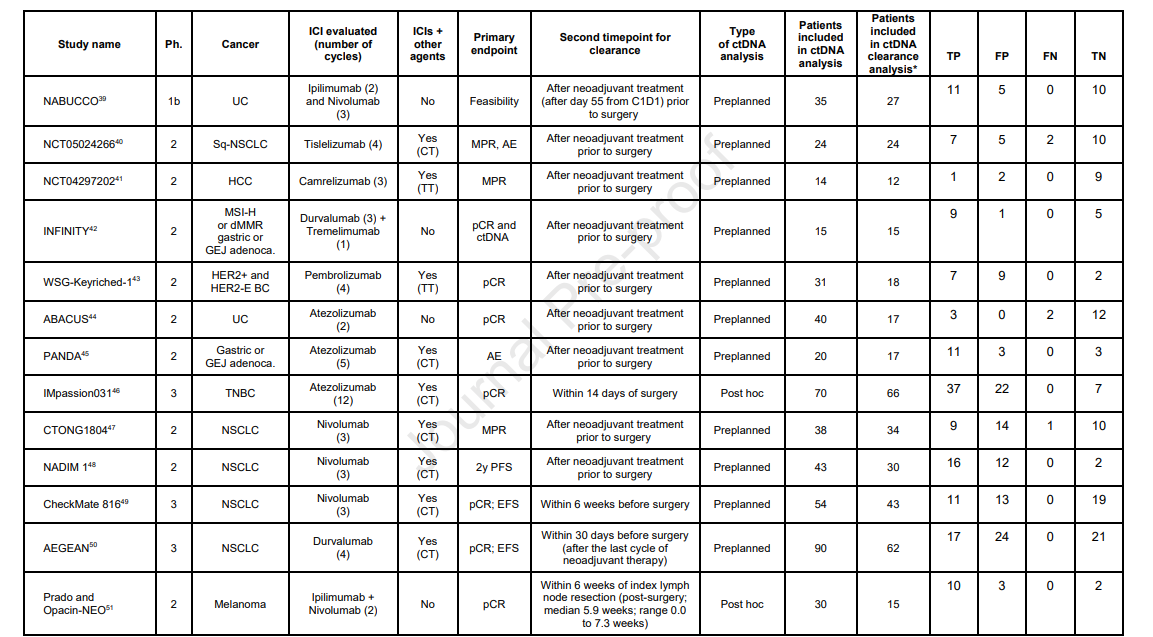
A comprehensive search was conducted across PubMed, EMBASE, and conference proceedings up to August 5, 2024. The inclusion criteria encompassed phase 1b, 2, or 3 clinical trials that investigated the relationship between ctDNA clearance and pCR in patients with solid tumors who had detectable ctDNA and were treated with neoadjuvant ICIs.
The primary objective was to determine the pooled sensitivity and specificity of ctDNA clearance in predicting pCR. Secondary metrics included positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR). A bivariate model was employed to compute these metrics, accompanied by 95% confidence intervals (CI).
Results
The analysis incorporated data from 13 trials, encompassing a total of 380 patients who had detectable ctDNA at baseline. Among these trials, 11 (85%) utilized a tumor-informed approach for ctDNA assessment. Key findings from the pooled data include:
- Pathologic Complete Response (pCR) Rate: 38% of patients achieved pCR.
ctDNA Clearance Rate: 73% of patients exhibited ctDNA clearance before or at the time of surgery. - Sensitivity: The pooled sensitivity was 0.98 (95% CI: 0.86–1.00), indicating that ctDNA clearance accurately identified 98% of patients who achieved pCR.
- Specificity: The pooled specificity was 0.53 (95% CI: 0.37–0.69), suggesting that ctDNA clearance correctly identified 53% of patients who did not achieve pCR.
- Positive Likelihood Ratio (PLR): Calculated at 2.09 (95% CI: 1.48–2.93), indicating that patients with ctDNA clearance are approximately twice as likely to achieve pCR compared to those without clearance.
- Negative Likelihood Ratio (NLR): Determined to be 0.04 (95% CI: 0.01–0.26), highlighting that the absence of ctDNA clearance is strongly associated with not achieving pCR.
- Diagnostic Odds Ratio (DOR): Found to be 57.36 (95% CI: 8.12–405.12), reflecting a substantial association between ctDNA clearance and pCR.
However, significant heterogeneity was observed across the studies (I² ≈ 70% for all metrics), indicating considerable variability in diagnostic performance.
Key Findings
The study underscores that while the absence of ctDNA clearance is a strong predictor for not achieving pCR, the presence of ctDNA clearance does not reliably confirm pCR due to its limited specificity. The high sensitivity suggests that ctDNA clearance is effective in identifying patients who will respond favorably to neoadjuvant ICI therapy.
However, the moderate specificity indicates that a significant proportion of patients with ctDNA clearance may not achieve pCR, leading to potential false-positive predictions. The notable heterogeneity across studies further complicates the interpretation, suggesting variability in ctDNA assessment methods and patient populations.
Key Takeaway Messages
While ctDNA clearance demonstrates potential as a predictive biomarker for pCR in patients with solid tumors undergoing neoadjuvant ICI therapy, its current utility is constrained by limited specificity and significant inter-study variability. The lack of ctDNA clearance may help identify patients unlikely to achieve pCR, but the presence of clearance does not guarantee a complete pathological response. Therefore, ctDNA clearance should not be used in isolation to guide clinical decisions.
Further research is warranted to standardize ctDNA assessment techniques and to validate its role in predicting treatment outcomes. Clinicians are advised to consider a comprehensive array of factors when formulating treatment plans, rather than relying solely on ctDNA clearance status
- Predictive Value of ctDNA Clearance: The absence of ctDNA clearance can be a useful indicator for identifying patients unlikely to achieve pCR.
- Limitations in Confirmatory Power: Due to limited specificity, ctDNA clearance alone should not be relied upon to confirm pCR.
- Need for Standardization: The observed heterogeneity highlights the necessity for standardized methodologies in ctDNA assessment to ensure consistent and reliable results.
- Clinical Decision-Making: Clinicians should exercise caution and not base treatment decisions solely on ctDNA clearance status in the neoadjuvant setting.
You can read the full article here
Written by Sona Karamyan, MD


