mRNA COVID-19 Vaccines have emerged as unexpected immune modulators capable of enhancing antitumor responses. While immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy, producing durable remissions across multiple tumor types, only a subset of patients experience long-term benefit because most solid tumors remain immunologically “cold,” lacking pre-existing antitumor immunity.
Traditionally, personalized mRNA cancer vaccines have been explored to sensitize tumors to ICIs through neoantigen-specific activation; however, their complexity and manufacturing time have limited widespread use. The present study demonstrates that clinically available SARS-CoV-2 mRNA vaccines can similarly act as potent immune activators that reprogram the tumor–immune interface and enhance responsiveness to checkpoint blockade—independent of tumor antigen targeting.
Mechanistic Insights
In preclinical melanoma and lung carcinoma models, SARS-CoV-2 spike mRNA–lipid nanoparticles (RNA-LNPs)induced a systemic surge in type I interferon (IFNα) and other antiviral cytokines, driving robust activation of antigen-presenting cells (APCs) such as dendritic cells and macrophages. This innate immune activation resulted in increased MHC-II expression, enhanced antigen presentation, and priming of tumor-reactive CD8⁺ T cells in lymphoid organs.
The effect was strictly dependent on IFNα signaling, as blockade of IFNAR1 completely abrogated anti-tumor activity, whereas IL-1 inhibition did not. IFN-driven innate immune activation also led to PD-L1 upregulation on tumor cells, suggesting that mRNA vaccines transform immunologically silent tumors into inflamed, PD-L1–expressing phenotypes that can subsequently respond to PD-1/PD-L1 blockade.
Combination therapy with RNA-LNPs and anti–PD-1 antibodies produced synergistic tumor regression in multiple mouse models, doubling intratumoral infiltration of tumor-specific CD8⁺ T cells expressing activation markers (PD-1, CD69, 4-1BB) and recognizing melanoma-associated antigens such as GP100, TRP2, WT1, survivin, and claudin-6. Notably, substituting the spike protein for non-tumor antigens (e.g., cytomegalovirus pp65) yielded similar results, indicating that the innate immunogenicity of mRNA and lipid nanoparticles—rather than the encoded antigen—drives the therapeutic effect.
Human Translational Correlates
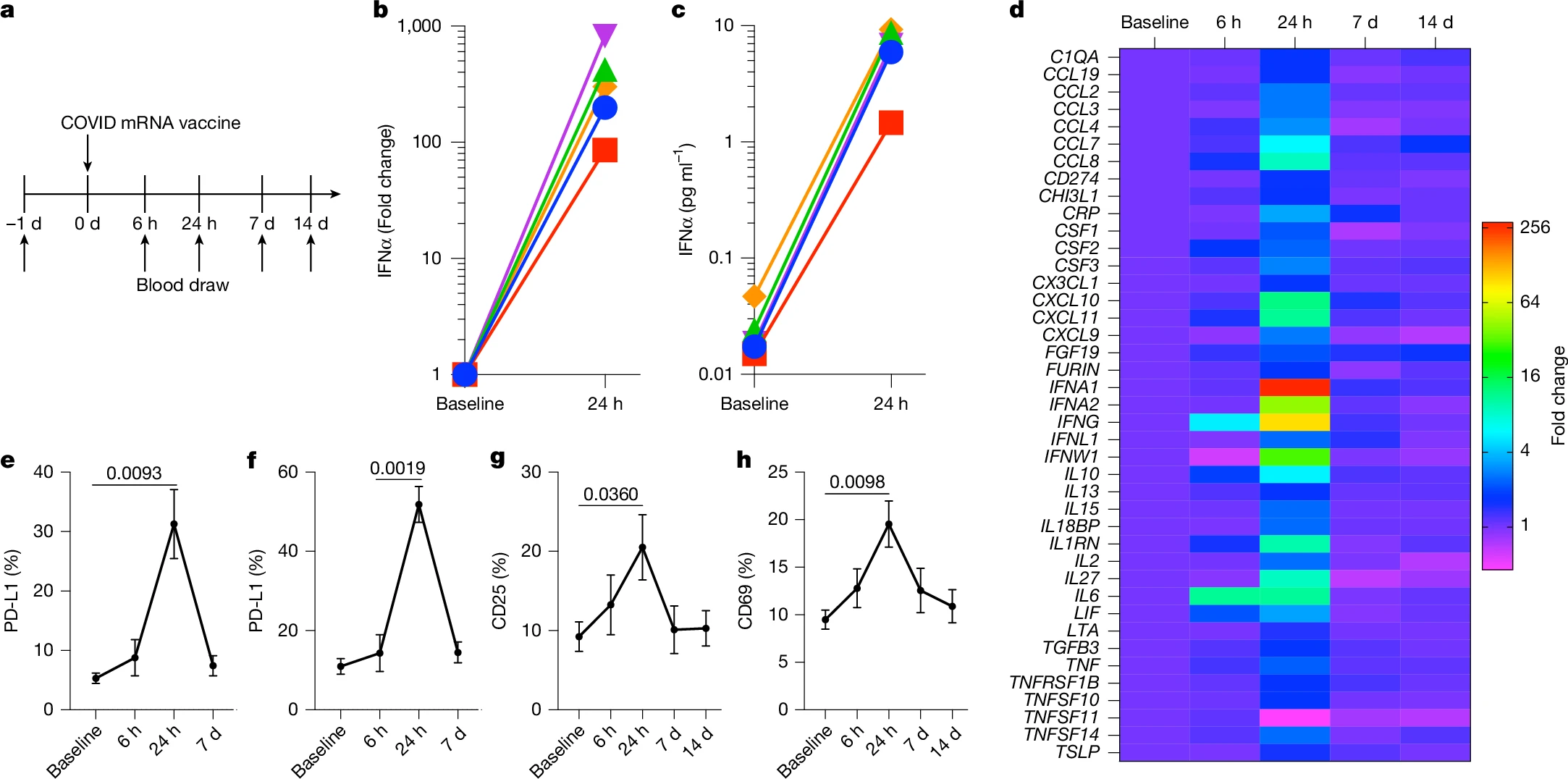
To confirm cross-species conservation, healthy human volunteers receiving mRNA-1273 (Moderna) or BNT162b2 (Pfizer-BioNTech) vaccines were analyzed longitudinally for cytokine and immune activation profiles. Within 24 hours of vaccination, plasma IFNα increased nearly 280-fold from baseline, accompanied by transient surges in IL-6, IFNγ, and CXCL10, as well as upregulation of PD-L1 on circulating CD11b⁺ myeloid cells and CD11c⁺ dendritic cells. NK cells and T cells displayed concurrent activation, evidenced by increased CD25 and CD69 expression, respectively. These effects resolved within seven days, consistent with transient but potent systemic immune reprogramming.
Clinical Findings
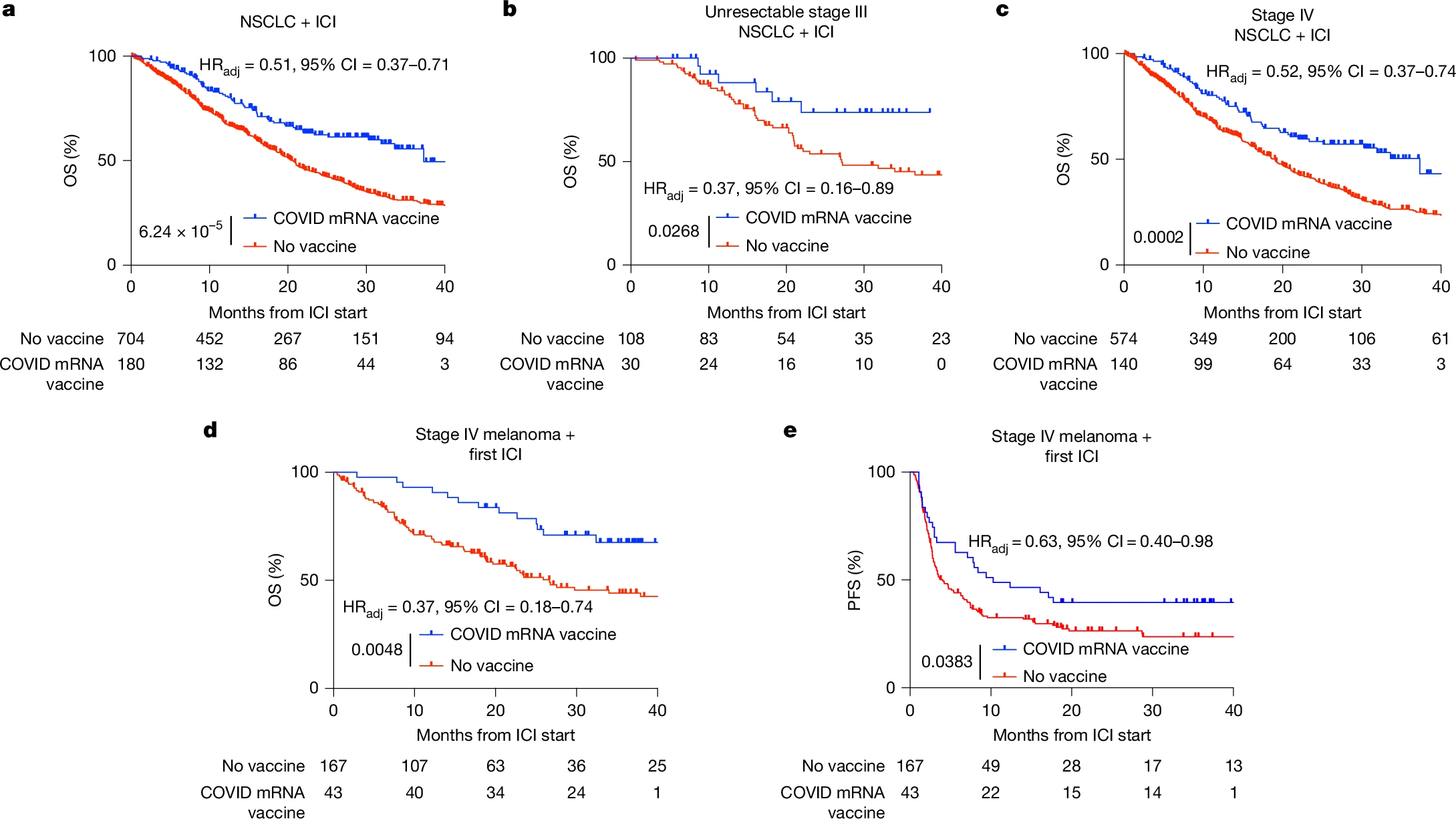
A retrospective analysis at The University of Texas MD Anderson Cancer Center examined over 1,000 patients with non–small cell lung cancer (NSCLC) or melanoma treated with ICIs between 2015 and 2022. Patients who received a COVID-19 mRNA vaccine within 100 days of ICI initiation experienced significantly prolonged overall survival (OS) and progression-free survival (PFS) compared to unvaccinated controls.
In NSCLC (n = 884), vaccinated patients had a median OS of 37.3 vs 20.6 months and 3-year OS of 55.7% vs 30.8% (HRadj = 0.51, 95% CI 0.37–0.71, p < 0.0001).
In metastatic melanoma (n = 210), vaccinated patients achieved median OS of 26.7 months vs unmet and HRadj = 0.37 (95% CI 0.18–0.74, p = 0.0048), with parallel improvements in PFS.
The survival benefit persisted after propensity score matching, time bias correction, and multivariable Cox regression controlling for 39 clinical covariates.
No survival advantage was observed in patients receiving other vaccines (influenza, pneumococcal) or in those treated with chemotherapy without ICIs, underscoring the specificity of the mRNA–ICI synergy.
Tumor Microenvironment Modulation
Pathologic analyses of 2,315 NSCLC biopsies revealed that patients vaccinated within 100 days before biopsy had significantly higher PD-L1 tumor proportion scores (TPS) (31% vs 25% in unvaccinated; p = 0.045), with a 29% greater likelihood of PD-L1 ≥50%, a key eligibility threshold for single-agent ICI therapy. A larger, tissue-agnostic cohort of 5,317 PD-L1–assessed samples showed a consistent 37% increase in PD-L1 TPS among recently vaccinated patients.
Importantly, patients with “cold” NSCLC (baseline PD-L1 <1%) who received an mRNA vaccine near ICI initiation exhibited OS comparable to PD-L1–high patients, indicating conversion of immune-resistant tumors into ICI-sensitive phenotypes.
Proposed Mechanism
The data support a model in which mRNA vaccines induce systemic innate immune activation via type I IFN, driving APC maturation and tumor antigen presentation. This primes effector T-cell responses, which upon infiltration, provoke tumoral PD-L1 upregulation. Subsequent PD-1/PD-L1 blockade sustains T-cell cytotoxicity, resulting in tumor regression and improved survival. This mechanistic cascade effectively “resets” the cancer–immune cycle, converting immunologically inert tumors into responsive ones.
Discussion and Implications
These findings reveal that clinically available, non–tumor-targeted mRNA vaccines can act as immune adjuvants for checkpoint blockade, offering a practical and widely accessible means to overcome intrinsic ICI resistance. The ability of mRNA–LNP formulations to elicit potent innate immune activation through MDA5-mediated sensing and IFN-I signaling provides a mechanistic basis for their synergy with ICIs.
Beyond their prophylactic use against infectious disease, off-the-shelf mRNA platforms may represent a new class of universal immunotherapy sensitizers, complementing or bridging access to personalized cancer vaccines. These results challenge conventional distinctions between antiviral and anticancer immunity, underscoring the shared molecular pathways that can be therapeutically exploited to enhance ICI efficacy.
You can read full article here


