Colorectal cancer prevention has traditionally rested on three pillars: population screening, lifestyle modification, and selective chemoprevention. While colonoscopy and fecal-based tests remain highly effective, global colorectal cancer incidence continues to rise, with a particularly concerning increase among younger adults. This epidemiologic shift has prompted renewed interest in systemic prevention strategies that target the biological drivers of colorectal carcinogenesis rather than relying solely on lesion detection.
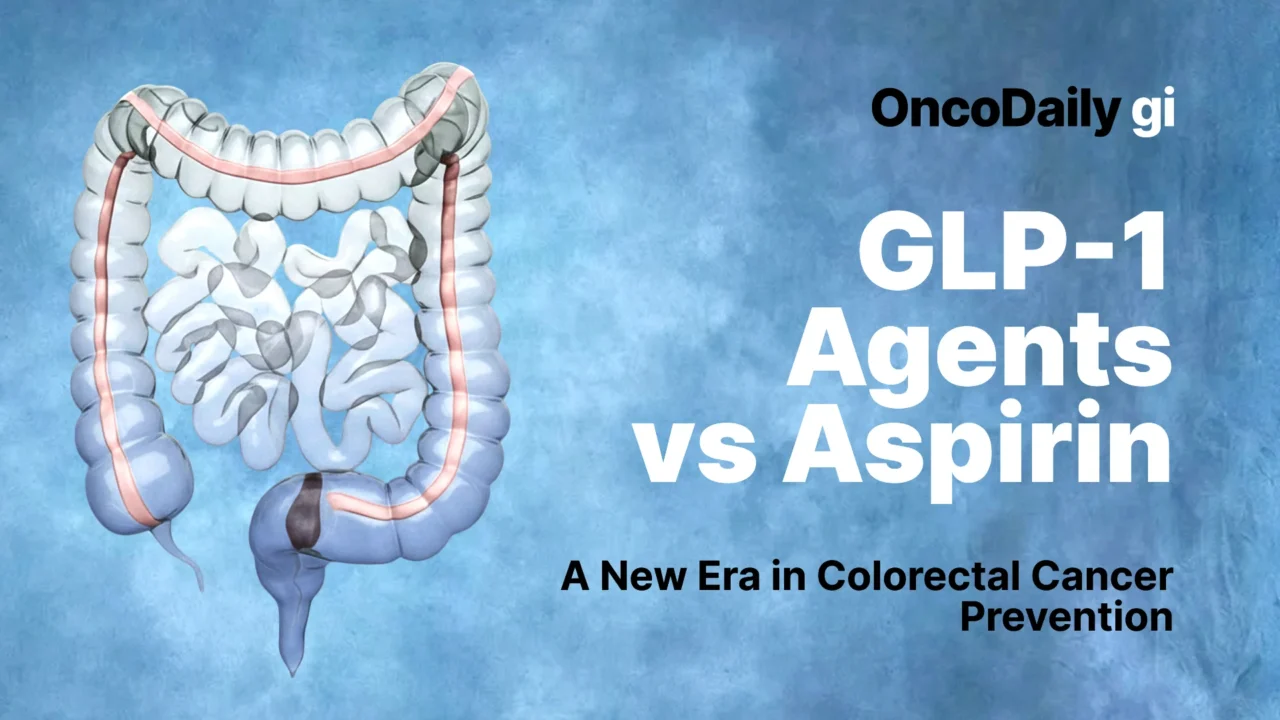
For decades, Aspirin has been the reference pharmacologic agent in colorectal cancer prevention. Its anti-inflammatory effects and long-term randomized evidence established the first era of chemoprevention. Today, however, emerging metabolic therapies—especially GLP-1 receptor agonists—are reshaping the conversation. New data presented at the ASCO Gastrointestinal Cancers Symposium 2026 have further strengthened the hypothesis that metabolic modulation may represent a new frontier in colorectal cancer prevention.
Aspirin and the First Era of Chemoprevention
Aspirin’s preventive effect in colorectal cancer is one of the most robust findings in cancer epidemiology. Through irreversible inhibition of cyclooxygenase enzymes, aspirin reduces prostaglandin-mediated inflammation, platelet activation, and downstream tumor-promoting signaling within the colorectal mucosa. Long-term follow-up of cardiovascular prevention trials first revealed lower colorectal cancer incidence and mortality among aspirin users.
Subsequent pooled analyses and randomized studies demonstrated that sustained aspirin use can reduce colorectal cancer incidence by approximately 20–30%, with benefits emerging after five or more years of continuous exposure. Importantly, the effect is most pronounced in proximal colon cancers, a region historically less protected by screening alone.
Despite its efficacy, aspirin’s role in average-risk colorectal cancer prevention has narrowed. Gastrointestinal bleeding, ulcer disease, and hemorrhagic stroke remain clinically significant risks, particularly in older adults. As a result, contemporary guidelines recommend aspirin only for carefully selected populations, such as individuals with elevated cardiovascular risk or hereditary colorectal cancer syndromes.
Metabolic Dysfunction as a Driver of Colorectal Cancer Risk
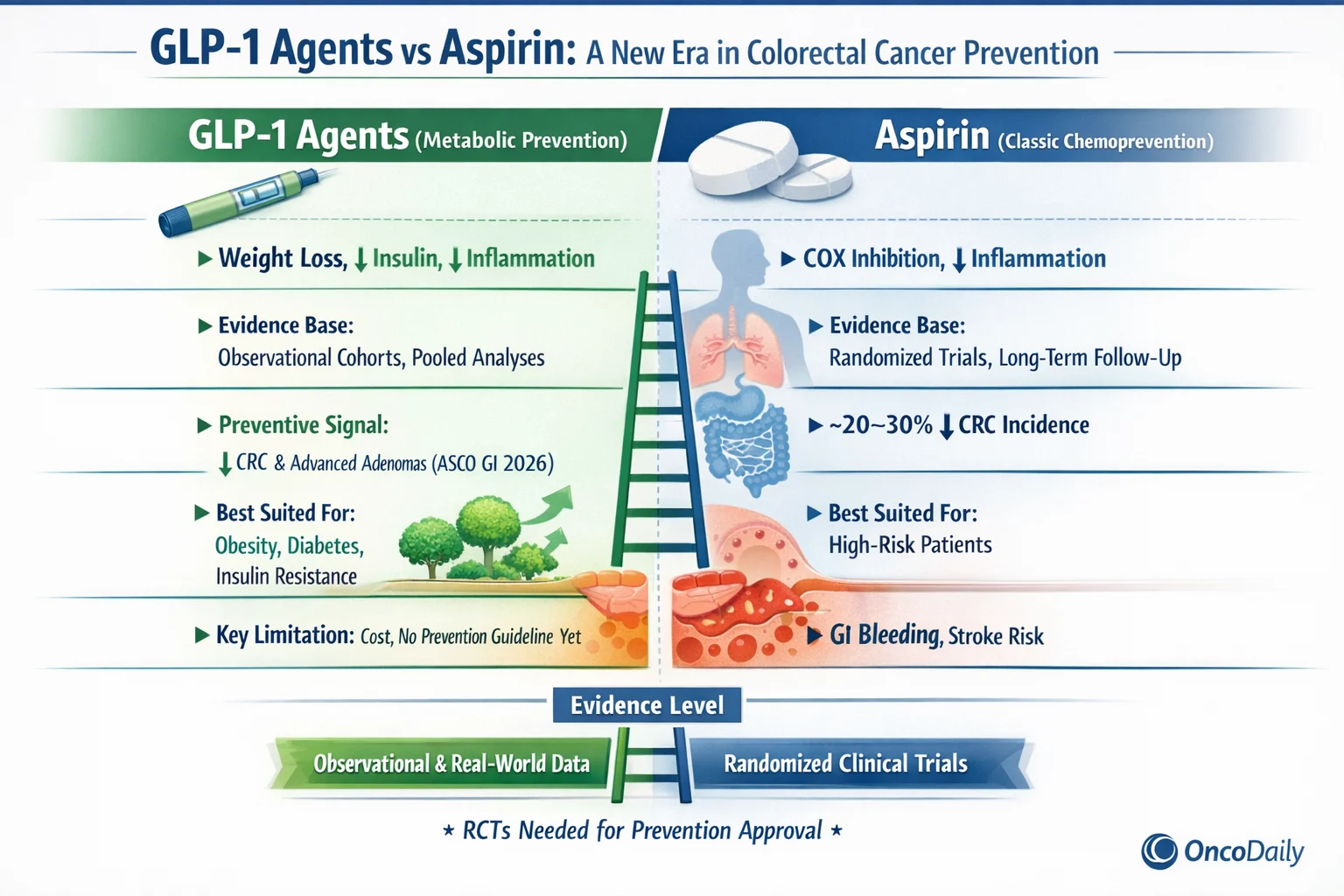
Over the past decade, the conceptual framework of colorectal cancer prevention has expanded to include metabolic health as a central determinant of cancer risk. Obesity, insulin resistance, type 2 diabetes, and visceral adiposity are now recognized as independent risk factors for colorectal adenomas and cancer.
Hyperinsulinemia and insulin-like growth factor signaling promote epithelial proliferation, inhibit apoptosis, and foster a pro-inflammatory microenvironment within the colon. These insights suggest that colorectal cancer prevention may be most effective when it targets upstream metabolic drivers rather than downstream inflammatory consequences alone.
GLP-1 Agents: From Diabetes Treatment to Preventive Signal
GLP-1 receptor agonists—such as Semaglutide, Liraglutide, and Dulaglutide—were originally developed for glycemic control in type 2 diabetes. Their clinical impact has since expanded dramatically due to their ability to induce sustained weight loss, improve insulin sensitivity, reduce systemic inflammation, and alter gut physiology.
These effects directly intersect with pathways implicated in colorectal carcinogenesis. As a result, investigators have increasingly explored whether GLP-1 therapy might confer protection against colorectal neoplasia, either as a direct biological effect or as a consequence of improved metabolic health.
Early observational studies suggested lower colorectal cancer incidence among patients treated with GLP-1 agents compared with those receiving other glucose-lowering therapies. While initially hypothesis-generating, these findings laid the groundwork for more comprehensive analyses.
What’s New: ASCO GI 2026 Data on GLP-1 Agents
At ASCO GI 2026, several large-scale analyses presented the strongest clinical signals to date linking GLP-1 receptor agonist use with reduced colorectal neoplasia risk. Importantly, these data did not originate from randomized colorectal cancer prevention trials. Instead, they were derived from large observational cohorts, retrospective comparative analyses, and pooled real-world datasets.
Across multiple national and multinational healthcare databases, GLP-1 use was associated with lower rates of colorectal cancer compared with non-use and with other antidiabetic therapies, including insulin and sulfonylureas. These associations persisted after adjustment for age, sex, body mass index, glycemic control, comorbidities, and screening intensity, suggesting that the observed effect extends beyond weight loss alone.
Additional ASCO GI 2026 analyses focused on adenoma recurrence, a validated surrogate endpoint in colorectal cancer prevention research. Patients receiving GLP-1 therapy demonstrated lower rates of advanced adenomas on surveillance colonoscopy, a finding particularly relevant given that adenoma prevention formed the evidentiary backbone for aspirin’s adoption in earlier prevention strategies.
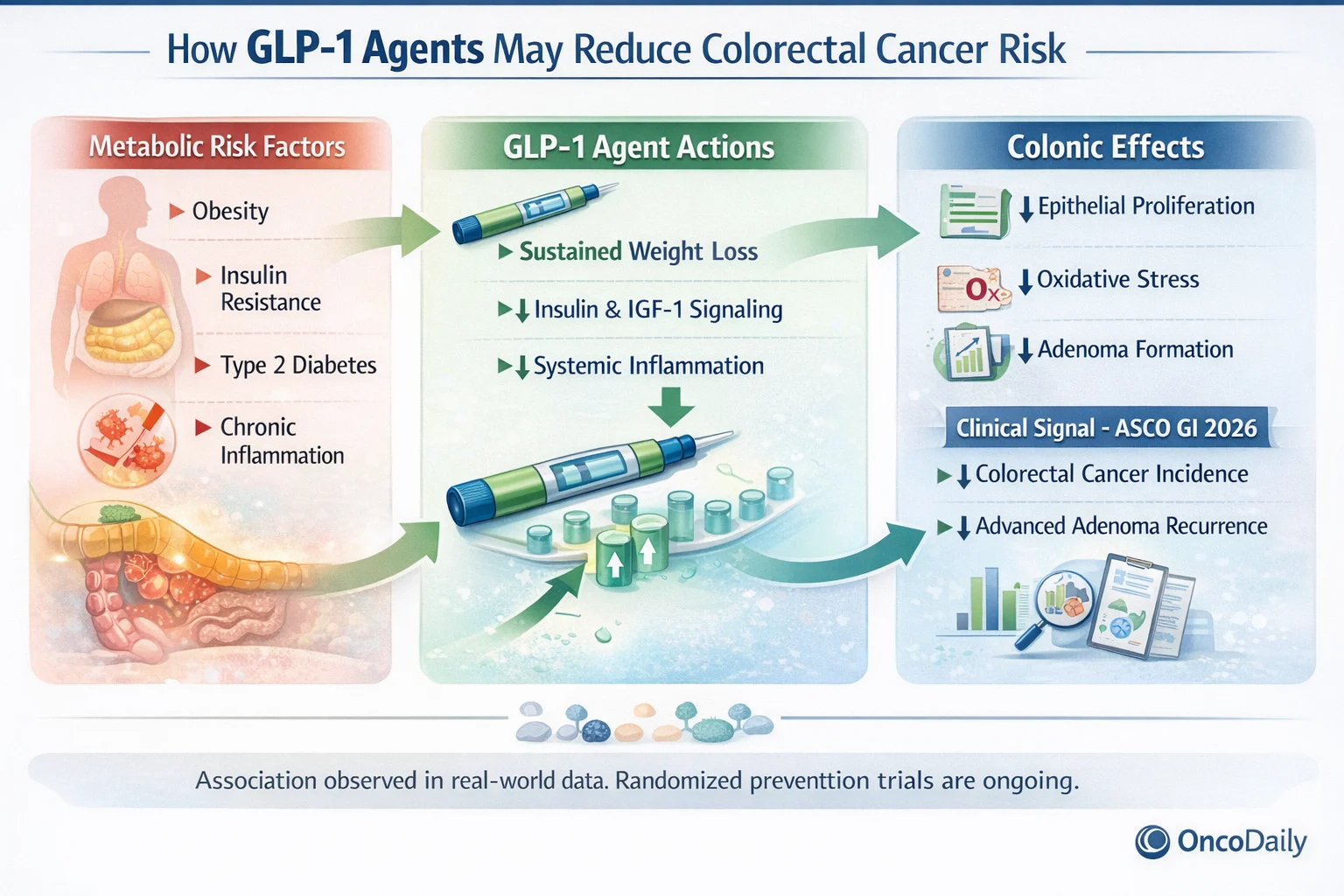
Evidence-Level Clarification: What These Data Do—and Do Not—Show
It is critical to frame the ASCO GI 2026 findings accurately.
The GLP-1 data presented to date are based on:
- Large observational cohort studies
- Retrospective comparative effectiveness analyses
- Meta-analytic pooling of observational datasets
They are not yet based on dedicated randomized clinical trials designed specifically to evaluate colorectal cancer incidence or adenoma prevention as primary endpoints. As such, the current evidence demonstrates association, not proven causality.
This contrasts with aspirin, whose preventive effect is supported by long-term randomized data with cancer-specific outcomes. Accordingly, GLP-1 agents are not currently recommended for colorectal cancer prevention in clinical guidelines. However, the consistency, magnitude, and biological plausibility of the ASCO GI 2026 signal strongly justify future randomized prevention trials.
Biological Plausibility: Why GLP-1 Agents May Reduce Risk
The preventive signal observed with GLP-1 therapy is biologically plausible and likely multifactorial. Beyond weight loss, GLP-1 agents reduce circulating insulin levels, attenuate insulin-like growth factor signaling, and lower systemic inflammatory markers. These changes directly counter pathways known to promote colorectal tumor initiation and progression.
Preclinical studies suggest that GLP-1 receptor signaling may also influence intestinal epithelial turnover, oxidative stress, and gut barrier integrity. Together, these effects point toward a systems-level preventive mechanism, in contrast to aspirin’s more focused anti-inflammatory action.
GLP-1 Agents vs Aspirin: A Contemporary Comparison
Viewed through a modern prevention lens, aspirin and GLP-1 agents represent two distinct strategies for colorectal cancer prevention. Aspirin is inexpensive, widely available, and supported by randomized evidence, but its bleeding risk limits universal use. GLP-1 agents, while costly and not yet guideline-endorsed for prevention, offer broader metabolic benefits without inherent bleeding risk.
The ASCO GI 2026 data suggest that GLP-1 therapy may be particularly relevant for metabolically high-risk populations, including individuals with obesity, insulin resistance, or type 2 diabetes—groups that now account for a substantial proportion of colorectal cancer burden.

Read About ALASCCA Trial Results on OncoDaily GI
Implications for Prevention Strategy and Public Health
If future randomized trials confirm these findings, colorectal cancer prevention may shift toward a risk-adapted, biologically informed model. Aspirin may retain a role in select inflammatory or hereditary risk settings, while GLP-1 agents could become foundational for metabolically driven risk reduction.
Such an approach aligns with modern public health priorities, offering the potential to reduce colorectal cancer incidence while simultaneously addressing obesity, diabetes, and cardiovascular disease.
Toward a Systems-Based Prevention Paradigm
Colorectal cancer prevention is evolving beyond single-agent chemoprevention. Aspirin defined the first era by targeting inflammation. Emerging evidence—strengthened by ASCO GI 2026—suggests that GLP-1 receptor agonists may define the next era by addressing the metabolic roots of colorectal carcinogenesis.
While randomized prevention trials are still needed, the consistency and biological coherence of current data support cautious optimism. The future of colorectal cancer prevention is likely to be integrated, metabolic, and personalized, reflecting a deeper understanding of cancer as a systemic disease.
You Can Watch More on OncoDaily Youtube TV