Colorectal cancer (CRC) remains one of the leading causes of cancer morbidity worldwide, with recurrence posing a major challenge even after curative surgery and adjuvant treatment. Aspirin has long been recognized for its preventive effect on colorectal adenomas and cancer, but its role in improving outcomes after diagnosis has been less well defined. Observational studies hinted that patients with PIK3CA-mutated tumors might derive a survival benefit from aspirin, yet randomized evidence was lacking.
The ALASCCA trial provides the first large-scale randomized data evaluating aspirin in a molecularly defined population of PI3K-altered localized CRC, offering new insights into precision prevention in the adjuvant setting.
What Is PI3K and Why Does It Matter in CRC?
Phosphoinositide 3-kinase (PI3K) is a key signaling enzyme that regulates cell growth, metabolism, survival, and proliferation. In healthy cells, PI3K activation is tightly controlled, but in many cancers — including colorectal cancer — this pathway becomes dysregulated.
Mutations in PIK3CA, the gene encoding the catalytic subunit p110α of PI3K, are among the most frequent genetic alterations in CRC. These mutations, especially in exons 9 and 20, cause constitutive activation of the PI3K–AKT–mTOR pathway, leading to uncontrolled tumor growth and resistance to programmed cell death. Because of its central role in oncogenesis, the PI3K pathway is a major focus of precision oncology.
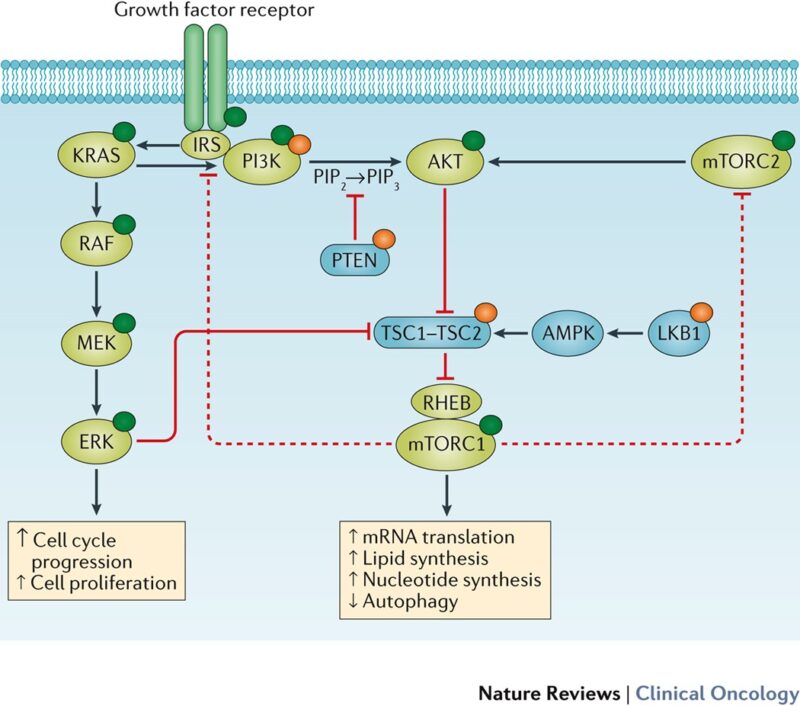
source: Nature Reviews Clinical Oncology
Connection Between Aspirin and the PI3K Pathway
Aspirin’s primary mechanism is the irreversible inhibition of cyclooxygenase (COX-1 and COX-2), leading to reduced synthesis of prostaglandin-endoperoxide synthase 2 (PTGS2, also known as COX-2) products such as prostaglandin E2 (PGE2). These prostaglandins promote inflammation and cell growth, and their reduction creates a tumor microenvironment less favorable to cancer progression.
According to a pivotal publication in The New England Journal of Medicine (Liao et al., 2012), this mechanism extends specifically to the PI3K–AKT–mTOR pathway. Aspirin’s COX-2 inhibition reduces PGE2 and indirectly down-regulates PI3K activity, cutting off one of the tumor’s key growth signals.
About the ALASCCA Trial
The ALASCCA (Aspirin for Localized Advanced Stage Colorectal Cancer with Altered PI3K Pathway) study was a double-blind, placebo-controlled, randomized clinical trial conducted across multiple centers in Sweden and other European countries. Eligible patients had stage I–III rectal cancer or stage II–III colon cancer harboring somatic alterations in PIK3CA, PIK3R1, or PTEN.
Participants were randomized 1:1 to receive 160 mg of aspirin daily or a matched placebo for three years. The study population was divided into two molecular subgroups:
- Group A: Patients with PIK3CA hotspot mutations (exons 9 or 20)
- Group B: Patients with other moderate- to high-impact PI3K pathway alterations
Endpoints and Study Design
The primary endpoint was time to colorectal cancer recurrence in Group A.
Secondary endpoints included recurrence in Group B, disease-free survival (DFS) across both groups, and safety outcomes.
Results
Results, published in the New England Journal of Medicine in September 2025, demonstrated a clinically significant reduction in recurrence with aspirin.
In Group A, the three-year cumulative incidence of recurrence was 7.7% with aspirin vs. 14.1% with placebo(hazard ratio [HR] 0.49; 95% CI 0.24–0.98; p=0.04).
In Group B, recurrence was 7.7% with aspirin vs. 16.8% with placebo (HR 0.42; 95% CI 0.21–0.83).
Three-year DFS improved from 81.4% to 88.5% in Group A and from 78.7% to 89.1% in Group B, with hazard ratios favoring aspirin.
Severe adverse events occurred more frequently with aspirin (16.8%) compared with placebo (11.6%), primarily related to bleeding and gastrointestinal toxicity. These findings provide the first randomized confirmation that low-dose aspirin nearly halves recurrence risk in patients with PI3K-altered CRC.
2025 Guideline Recommendation: Is Aspirin Now Advised for PI3K-Mutated CRC?
In line with the latest NCCN Guidelines (Version 4.2025), there is now a formal recommendation to consider PIK3CA testing for stage II–III colon cancer. For patients found to have a PIK3CA mutation, the guidelines specify that low-dose aspirin (100–162 mg orally once daily) should be administered for 3 years as part of adjuvant management.
This update reflects growing evidence—including the ALASCCA trial results published in NEJM (September 2025)—that adjuvant aspirin therapy significantly reduces recurrence risk in patients with PI3K-pathway–altered colorectal cancer.

Conclusion
The ALASCCA trial marks a milestone in precision adjuvant therapy for colorectal cancer. Low-dose aspirin, a widely available and inexpensive drug, significantly improved outcomes in patients with PIK3CA and other PI3K pathway alterations. Although an increased risk of severe adverse events was observed, the absolute reduction in recurrence rates and improvement in DFS highlight aspirin as a promising strategy for selected patients.
Future work will focus on refining molecular selection, balancing benefit against bleeding risk, and integrating aspirin into clinical practice guidelines for PI3K-altered CRC.


