Gastroesophageal adenocarcinoma (GEA) is a biologically heterogeneous malignancy with a poor prognosis in advanced stages. Over the past decade, biomarker-driven strategies—most notably targeting HER2 amplification, mismatch repair deficiency, and PD-L1 expression—have progressively refined therapeutic decision-making. More recently, Claudin 18.2 (CLDN18.2) has emerged as a clinically relevant biomarker following positive phase III trials evaluating zolbetuximab in combination with chemotherapy.
CLDN18.2 is physiologically expressed in epithelial cells of the normal gastric mucosa but becomes aberrantly exposed on the surface of malignant cells during oncogenic transformation, rendering it accessible for antibody-based therapeutic targeting. While pivotal clinical trials have reported CLDN18.2 positivity in approximately 24–33% of advanced GEA, its real-world prevalence, distribution across molecular subtypes, and association with treatment outcomes—particularly in Western populations—remain incompletely characterized.
This ambispective, multicenter European study aimed to comprehensively characterize CLDN18.2 expression in a large cohort of patients with advanced GEA, evaluate its overlap with established biomarkers, and assess clinical outcomes according to CLDN18.2 status across standard treatment strategies.
Title: Claudin 18.2 expression in gastroesophageal adenocarcinoma: biomarker overlap and association with clinical outcomes in a European cohort
Authors: E. Terán, R. Pazo, L. Caritá, M. Alsina, C. Hierro, C. Blanco, M. Reboredo, S. Landolfi, A. Zucchiatti, L. Visa, A. Calvo, A. López, R. Vidal-Tocino, C. Agra, I. Alés, S. Foti, B. García-Paredes, V. Genovesi, L. Fornaro, T. Sauri, I. Macías, E. Martínez, J. Martínez, C. Buges, P. Ribera, J.M. Herranz, D. Acosta, A. Vivancos, J. Tabernero, E. Élez, T.V. Tian, T. Macarulla

Read about Zolbetuximab: Uses in Cancer, Side Effects, Dosages, Expectations on OncoDaily.
Methods
This ambispective, multicenter study included 563 patients with locally advanced unresectable or metastatic gastroesophageal adenocarcinoma diagnosed between June 2019–May 2025 across 17 European institutions (Spain and Italy). Archival tumor samples from primary tumors or metastatic sites were assessed for CLDN18.2 expression using immunohistochemistry (IHC).
CLDN18.2-high expression was defined as moderate-to-strong (2+/3+) membranous staining in ≥75% of tumor cells, while CLDN18.2-low expression corresponded to staining in <75% of tumor cells.
Additional biomarker assessments included HER2 status, mismatch repair (MMR) proteins, Epstein–Barr virus (EBV), and PD-L1 expression using combined positive score (CPS). DNA- and RNA-based next-generation sequencing (NGS) was performed in a subset of patients to characterize genomic alterations and detect CLDN18–ARHGAP26/6 fusions.
Clinical outcomes included overall response rate (ORR), median progression-free survival (mPFS), and median overall survival (mOS) according to CLDN18.2 expression and treatment strategy. Survival analyses were performed using Kaplan–Meier estimates and Cox proportional hazards models.
Results
High CLDN18.2 expression was observed in 48.3% of the overall cohort. CLDN18.2-high tumors were significantly associated with diffuse-type histology, the presence of signet-ring cells, and peritoneal metastases, and were less frequently associated with liver metastases. No significant differences were observed according to sex, ethnicity, tissue sampling site, or sample age.
Biomarker overlap
Within each subgroup, the proportion of tumors that were CLDN18.2-high was 33.9% in dMMR, 62.5% in EBV-positive, and 41.9% in HER2-positive disease. Among PD-L1–positive tumors, CLDN18.2-high was present in 45.7% of CPS ≥1 cases and 47.2% of CPS ≥5 cases
No statistically significant differences in PD-L1 CPS distribution were observed between CLDN18.2-high and CLDN18.2-low tumors at CPS cut-offs of 1, 5, or 10.
Overall, 21.5% of patients demonstrated overlap between CLDN18.2-high expression and at least one additional biomarker (HER2 positivity, PD-L1 CPS ≥5, EBV positivity, or dMMR). Coexpression of three biomarkers was observed in 17 patients: CLDN18.2/PD-L1/EBV (n=1), CLDN18.2/PD-L1/HER2 (n=10), and CLDN18.2/PD-L1/dMMR (n=6).
Read about CLDN18.2 and PD-L1 Expression Reveal Distinct Molecular Pathways and Therapeutic Implications in Advanced Gastric and GEJ Cancer on OncoDaily.
Genomic characteristics
Among patients who underwent next-generation sequencing, APC mutations and CDK6 amplifications were more frequently observed in CLDN18.2-low tumors. NF1 mutations were more prevalent in CLDN18.2-high tumors after exclusion of EBV-positive, dMMR, and HER2-positive cases. CLDN18–ARHGAP26/6 fusions were identified in 2 of 123 patients (1.6%), both diffuse-type with peritoneal involvement.
Clinical outcomes by treatment strategy
CLDN18.2 expression was not associated with differences in ORR, mPFS, or mOS in patients treated with fluoropyrimidine–platinum doublet chemotherapy.
HER2-targeted therapy
Among patients receiving first-line chemotherapy plus HER2-targeted therapy, CLDN18.2-high expression was associated with:
- Significantly longer mPFS (17.0 vs 8.9 months; HR 0.42; P = 0.03)
- Numerically longer mOS (43.0 vs 23.0 months; HR 0.40; P = 0.07)
Immunotherapy-based regimens
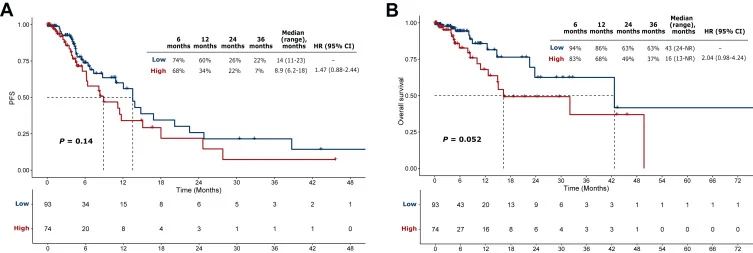
In patients treated with immunotherapy in first- or second-line settings, CLDN18.2-high expression showed a trend toward:
- Lower ORR
- Numerically shorter mPFS and mOS
These findings reflected a trend toward poorer outcomes across PD-L1 CPS cut-offs and became more pronounced at higher CLDN18.2 expression thresholds. In univariate analysis, dMMR/MSI-H status was associated with improved mOS, while in multivariate analysis, CLDN18.2-high status tended to correlate with worse mOS.
Conclusion
In patients receiving first-line fluoropyrimidine–platinum chemotherapy, CLDN18.2 status was not associated with ORR, mPFS, or mOS. In contrast, CLDN18.2-high expression was associated with longer progression-free survival in patients receiving HER2-targeted therapy and showed a trend toward poorer outcomes in patients treated with immunotherapy-based regimens.
These findings underscore the complexity of biomarker overlap in advanced GEA and highlight the need for prospective, biomarker-driven studies to optimize treatment selection and sequencing in this heterogeneous disease.
The full study is available in ESMO Open.