Gastric and GEJ (gastroesophageal junction) adenocarcinomas remain among the deadliest malignancies globally, responsible for nearly 800,000 deaths each year. Despite therapeutic advances with immune checkpoint inhibitors and targeted agents, survival in metastatic disease remains limited.
Two biomarkers—Claudin 18.2 (CLDN18.2) and programmed death-ligand 1 (PD-L1)—have become central to precision treatment in gastric cancer. CLDN18.2, a tight-junction protein normally confined to gastric mucosa, becomes exposed during malignant transformation and is targetable by zolbetuximab. PD-L1 expression, quantified by the combined positive score (CPS), predicts benefit from immunotherapy. Yet, how these biomarkers interact and influence disease biology is not fully understood.
This study investigated the relationship between CLDN18.2 and PD-L1 expression in advanced microsatellite-stable (MSS), HER2-negative gastric and GEJ adenocarcinomas treated with first-line chemoimmunotherapy, analyzing correlations with molecular alterations, clinicopathologic features, and survival outcomes.
Study Design and Endpoints
This retrospective cohort included 70 patients treated at the City of Hope National Medical Center between 2020 and 2024. All received a fluoropyrimidine–platinum backbone combined with nivolumab or pembrolizumab.
- CLDN18.2 expression was assessed by immunohistochemistry (moderate-to-strong staining in ≥75% of tumor cells defined positivity).
- PD-L1 CPS was determined using the 22C3 antibody, with CPS ≥1 considered positive.
- Genomic profiling employed a 720-gene hybrid DNA/RNA sequencing panel to identify alterations in TP53, KRAS, CDH1, PIK3CA, RHOA, POLE, and CLDN18–ARHGAP6 fusions.
Primary endpoints were overall survival (OS) and progression-free survival (PFS); secondary analyses examined molecular correlates, metastatic distribution, and independent prognostic factors via Cox regression.

You can also read about Zolbetuximab: Uses in Cancer, Side Effects, Dosages, Expectations, and More on OncoDaily.
Results
The findings, published in ESMO Gastrointestinal Oncology (2025), identified two biologically distinct—but prognostically similar—subgroups based on CLDN18.2 and PD-L1 expression.
- CLDN18.2-positive tumors were more often diffuse-type (84%) and gastric in origin (87%), while CLDN18.2-negative tumors were enriched for GEJ cancers (44%) and KRAS mutations.
- PD-L1 expression tended to be higher in CLDN18.2-negative tumors (mean CPS = 14.0) versus CLDN18.2-positive (mean CPS = 5.4), reflecting an inverse relationship between these markers.
- Co-occurrence of CLDN18.2 and high PD-L1 CPS ≥ 10 was rare.
Genomic profiling showed TP53 mutations were frequent in both groups. CDH1 and RHOA alterations predominated in CLDN18.2-positive tumors, consistent with diffuse histology, whereas KRAS mutations were more common in CLDN18.2-negative tumors, possibly linking this subset to enhanced immune activity. The CLDN18–ARHGAP26/6fusion appeared almost exclusively in CLDN18.2-positive tumors.
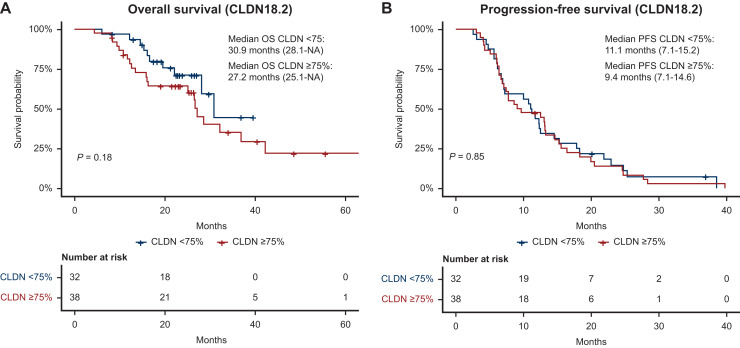
No significant survival differences were seen between groups: median OS was 27.2 months for CLDN18.2-positive versus 30.9 months for CLDN18.2-negative tumors; median PFS was 9.4 vs 11.1 months, respectively. Metastatic burden emerged as a key determinant of outcome—patients with oligometastatic disease had longer OS (23.2 months) compared with those with polymetastatic spread (14.6 months).
Ten patients with peritoneal metastases underwent hyperthermic intraperitoneal chemotherapy (HIPEC). While HIPEC did not significantly improve OS or PFS, a numerical trend favored those with low-volume disease, supporting further investigation in selected patients.
Conclusion
This analysis demonstrates that CLDN18.2 and PD-L1 expression define distinct molecular and immunologic subtypes of advanced gastric and GEJ adenocarcinoma.
CLDN18.2-positive tumors were characterized by diffuse histology, CDH1 and RHOA alterations, and low PD-L1 expression—hallmarks of an immune-cold environment. In contrast, CLDN18.2-negative tumors exhibited higher PD-L1 CPS, KRAS mutations, and intestinal morphology, aligning with an immune-active phenotype that may respond more favorably to checkpoint blockade.
Despite these biological distinctions, neither CLDN18.2 nor PD-L1 independently predicted survival, reinforcing that their greatest utility lies in therapeutic stratification rather than prognosis. The lack of clear benefit from HIPEC further emphasizes the importance of precise patient selection and biomarker integration in multimodal care.
Future trials should explore optimal sequencing or combination strategies for CLDN18.2-targeted and PD-L1-directed therapies, integrating dynamic biomarker monitoring, multi-site tumor sampling, and regional treatment approaches to advance personalized oncology.


