At the ESMO Congress 2025 in Berlin, Dr. Clara Montagut (Barcelona, Spain) presented the final results of the phase II CITRIC trial (LBA33) — a multicentre, randomized study exploring whether ctDNA-guided molecular selection can optimize rechallenge with anti-EGFR therapy in RAS/BRAF wild-type metastatic colorectal cancer (mCRC).
At OncoDaily GI, we spotlight the innovations reshaping gastrointestinal oncology — from ctDNA-driven treatment sequencing to targeted therapy rechallenge strategies redefining metastatic care.
Background
In metastatic colorectal cancer, resistance inevitably develops after first-line treatment with oxaliplatin- and irinotecan-based chemotherapy combined with targeted agents. Once patients progress beyond second line, available options such as trifluridine/tipiracil (FTD/TPI) or regorafenib yield limited benefit, with response rates often below 5% and median progression-free survival (PFS) around two to three months.
Rechallenge with anti-EGFR therapy — using agents like cetuximab or panitumumab — has emerged as a potential strategy to recapture sensitivity in selected patients who previously responded. However, resistance-driving mutations in RAS, BRAF, or EGFR-ECD frequently appear after exposure, making molecular selection critical.
The CITRIC trial was therefore designed to test a liquid biopsy–based hyperselection approach, using circulating tumor DNA (ctDNA) to identify patients who remain molecularly wild-type and likely to benefit from EGFR blockade rechallenge.
Trial Design
CITRIC (EudraCT 2020-000443-31) was a randomized, open-label, multicentre phase II study enrolling patients with RAS wild-type mCRC who had achieved prior benefit from a first-line anti-EGFR–containing regimen, followed by progression on a second-line regimen that did not include EGFR inhibition.
Before randomization, patients underwent ctDNA analysis using the Oncomine Colon cfDNA Assay (Thermo Fisher). Only those with RAS, BRAF, and EGFR-ECD wild-type ctDNA were eligible. Participants were then randomized 1:1 to receive either cetuximab plus irinotecan (CET+IRI) or investigator’s choice (IC) of standard non-EGFR-based therapies, including FTD/TPI±bevacizumab, fluoropyrimidine±bevacizumab, or regorafenib.

The primary endpoint was objective response rate (ORR) according to RECIST v1.1.
Secondary endpoints included disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and safety. An exploratory analysis using extended ctDNA profiling (MSK-ACCESS panel) was conducted to evaluate predictive biomarkers of response.
Results from ESMO 2024
Preliminary results from CITRIC, presented at ESMO 2024 and published in Annals of Oncology (2024), offered the first signal of efficacy for ctDNA-guided anti-EGFR rechallenge.
Between August 2020 and February 2024, 114 patients were screened, of whom 28 (25%) harbored RAS/BRAF/EGFR-ECD mutations and were excluded. Fifty-eight patients were randomized — 31 to CET+IRI and 27 to IC. Median age was 61.9 years, and nearly 40% were female.
After a median follow-up of 5.1 months, partial responses were observed in 3 of 31 patients (9.7%) in the cetuximab arm and none in the control arm. The disease control rate was 71% vs 33% (p=0.008), and the median treatment duration was significantly longer in the cetuximab arm (3.8 vs 2.1 months; p=0.001). Toxicities were manageable and consistent with prior anti-EGFR experience, primarily skin rash. These early findings suggested that ctDNA-based molecular selection could identify a subgroup of mCRC patients who retain sensitivity to EGFR inhibition even after previous exposure — laying the foundation for the definitive 2025 analysis.
Results from ESMO 2025
In the final 2025 readout, 114 patients were screened by ctDNA; 28 (25%) carried RAS/BRAF/EGFR-ECD mutations and were excluded, underscoring the importance of molecular selection.
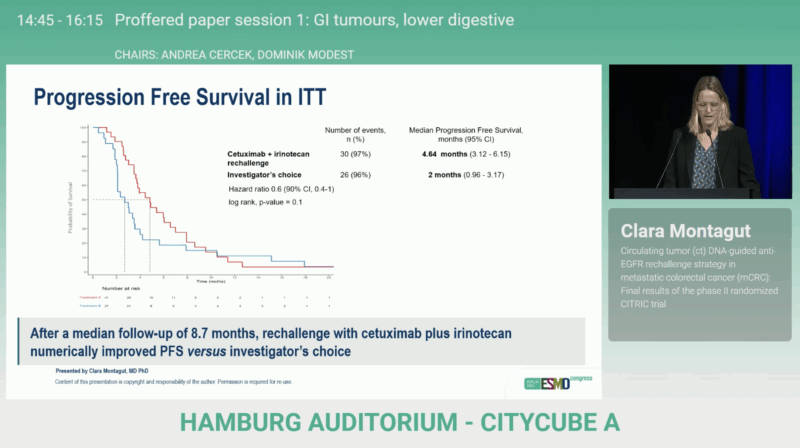
Among the 58 randomized patients (31 in CET+IRI, 27 in IC), cetuximab plus irinotecan achieved a disease control rate of 78% versus 44%, with median PFS of 4.64 months vs 2 months and median OS of 11.3 vs 7.3 months. While the difference in objective response rate remained modest, the consistency of DCR and PFS benefit demonstrated a clear clinical signal favoring rechallenge.
Crucially, ctDNA hyperselection proved predictive of benefit: patients with no detectable resistance mutations had PFS of 4.9 months vs 2.8 months in ctDNA-positive cases (p=0.01). Adverse events mirrored prior cetuximab experience, primarily skin toxicity, without unexpected safety signals.
Median follow-up was 8.7 months, with crossover to anti-EGFR therapy permitted in both arms.
Conclusions
The CITRIC trial is the first randomized study to validate ctDNA-guided anti-EGFR rechallenge as a feasible and effective third-line strategy in mCRC.
By excluding patients with acquired resistance and selecting only those with RAS/BRAF/EGFR-ECD wild-type ctDNA, the trial achieved clinically meaningful improvements in disease control and progression-free survival, reaffirming the potential of liquid biopsy to extend the benefit of precision-targeted therapy.
These findings position ctDNA-based selection as a cornerstone in the evolving landscape of treatment sequencing for metastatic colorectal cancer — offering a personalized, biomarker-driven path forward where therapeutic options remain limited.
You can read the full abstract here.