Stage III resectable NSCLC—especially N2 disease (ipsilateral mediastinal/subcarinal nodes) and multistation N2—has historically carried a poor prognosis, with high post-surgical relapse rates. CheckMate 77T previously established a perioperative benefit for neoadjuvant nivolumab + chemotherapy → surgery → adjuvant nivolumab versus chemo + placebo. This exploratory analysis asks a practical question: does baseline nodal status (N2 vs non-N2) still define “bad biology” in the era of perioperative immunotherapy?
Study Design and Methods
This report is an exploratory subgroup analysis of the randomized, double-blind phase 3 CheckMate 77T trial, focused specifically on stage III resectable NSCLC and outcomes by baseline nodal status.
Population (stage III only, stratified by nodal category):
- N2 disease: nivolumab arm n=91 vs placebo arm n=90
- Stage III non-N2 disease: nivolumab arm n=55 vs placebo arm n=57
- Patients with stage III N3 were excluded from this analysis because they were considered unresectable.
Treatment strategy (perioperative approach):
- Experimental arm: neoadjuvant nivolumab 360 mg plus chemotherapy every 3 weeks for 4 cycles, followed by surgery, then adjuvant nivolumab 480 mg every 4 weeks for up to 13 cycles.
- Control arm: matched placebo plus chemotherapy → surgery → adjuvant placebo.
What was assessed (key endpoints for this nodal analysis):
- Event-free survival (EFS) from randomization, with additional surgery-landmarked EFS analyses
Pathologic response after resection:
- pCR: 0% viable tumor in the lung and sampled lymph nodes
- MPR: ≤10% viable tumor in the lung and nodes
- Surgical outcomes: rates of definitive surgery, procedure type (lobectomy vs pneumonectomy), and R0 resection
- Downstaging metrics: nodal and primary tumor downstaging from baseline clinical stage to postsurgical pathologic stage
Follow-up:
- Median follow-up was approximately 25 months, based on the September 6, 2023 database lock.
Results Overview
N2 disease: a meaningful shift in a historically high-risk population
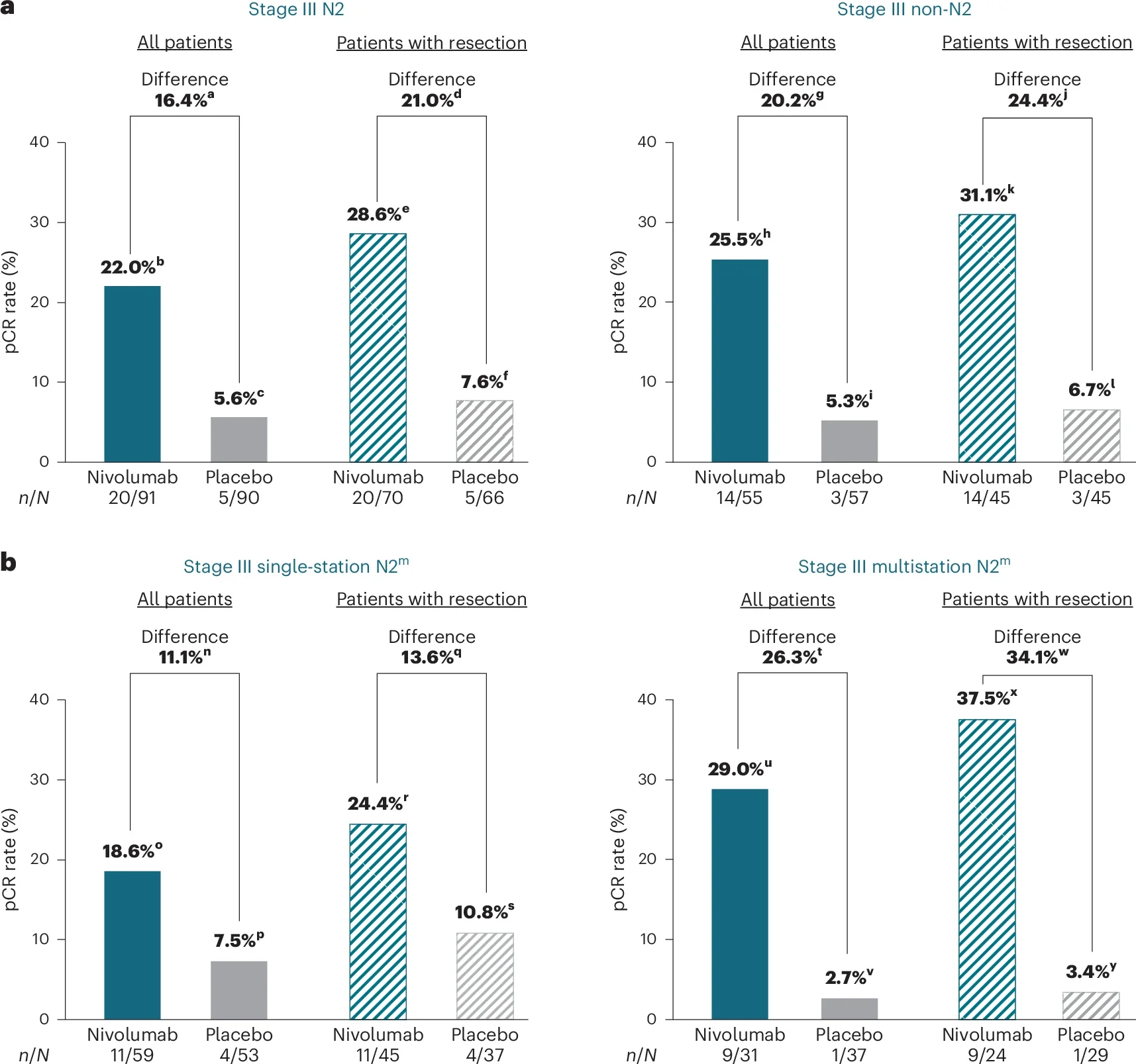
Perioperative nivolumab delivered a clear and clinically relevant improvement in outcomes among patients with baseline N2 disease. Event-free survival (EFS) at 1 year was 70% with nivolumab versus 45% with placebo, translating into a 54% reduction in risk of events (HR 0.46; 95% CI 0.30–0.70). Median EFS improved strikingly from 10.0 months to 30.2 months. Pathologic responses mirrored these gains, with pCR rates rising to 22.0% compared with 5.6% on placebo; MPR rates similarly favored the nivolumab arm.
Multistation N2: benefit persists despite adverse nodal anatomy
Even in patients with multistation N2—often considered the most unfavorable subset—the immunotherapy signal remained robust. One-year EFS reached 71% versus 46% (HR 0.43; 95% CI 0.21–0.88), and pCR increased to 29.0%compared with 2.7% on placebo. Importantly, the extent of nodal involvement did not negate benefit.
Non-N2 stage III: favorable trends with statistical uncertainty
Among patients without N2 disease, outcomes consistently favored nivolumab, though confidence intervals reflected smaller numbers. One-year EFS was 74% with nivolumab versus 62% with placebo (HR 0.60; 95% CI 0.33–1.08). Median EFS was not reached in the nivolumab arm versus 17.0 months with placebo, supporting a positive direction of effect without definitive statistical separation.
Surgical outcomes: preserved feasibility and less extensive resections
Definitive surgery rates remained high across arms in N2 disease (77% with nivolumab vs 73% with placebo). Among operated patients, lobectomy was more frequent with nivolumab (84% vs 74%), while pneumonectomy was markedly less common (1% vs 14%). R0 resection rates were comparable (~86% in both arms). Collectively, these data suggest improved operability and a shift toward less radical surgery with perioperative nivolumab plus chemotherapy.
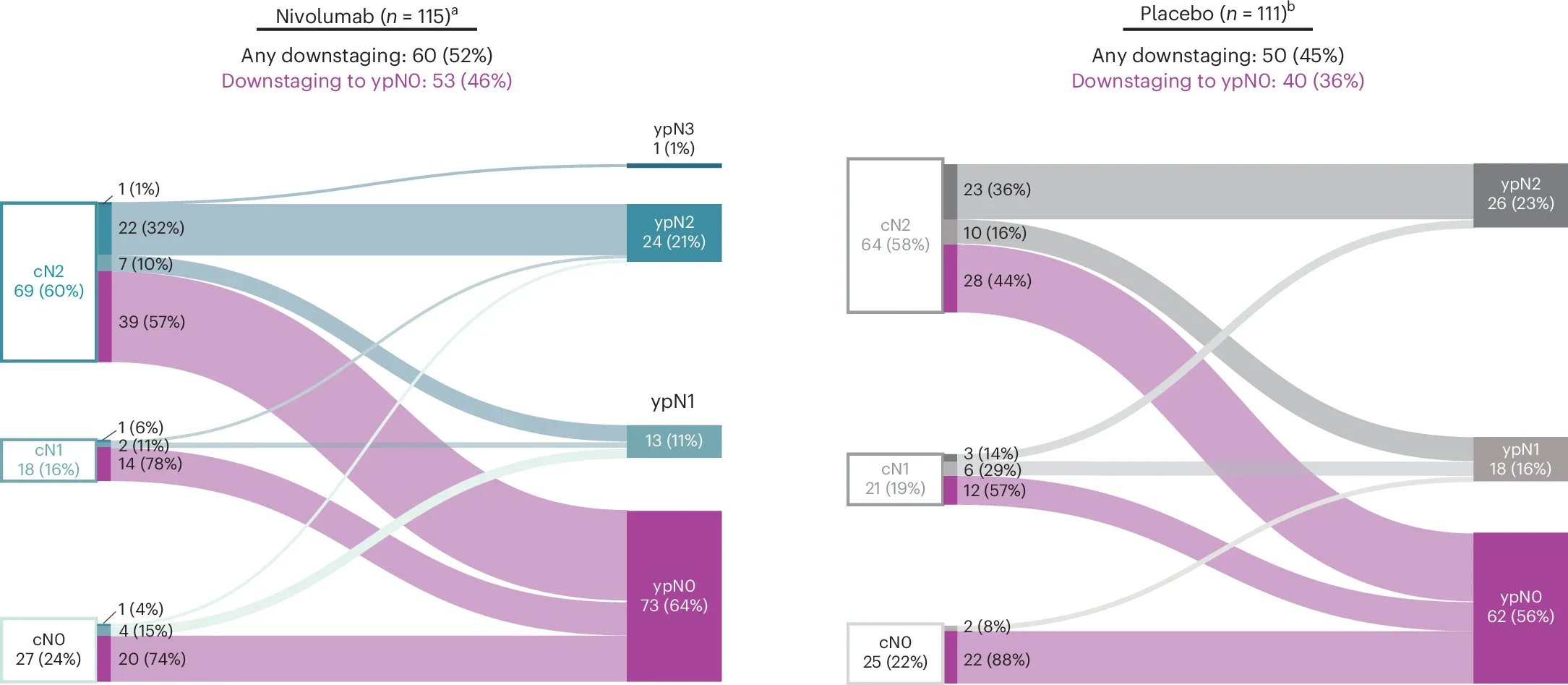
Downstaging: enhanced nodal clearance
In baseline cN2 patients who underwent surgery, nodal downstaging occurred in 67% of those receiving nivolumab versus 59% with placebo. Conversion to ypN0 was also more frequent (57% vs 44%), underscoring effective nodal clearance.
Safety: expected profile with clinically relevant immune risk
Safety findings aligned with known nivolumab toxicities. In the N2 subgroup, treatment discontinuation due to adverse events was higher with nivolumab, and two treatment-related deaths (pneumonitis) were reported—highlighting that while efficacy is substantial, immune-related risks remain clinically significant.
Insights (Why this matters)
- N2 has historically been a “poor prognosis” label, often pushing clinicians toward definitive chemoradiation rather than surgery in many scenarios.
- In this dataset, perioperative nivolumab + chemo meaningfully improves EFS and pCR in N2, including multistation N2, and surgical outcomes remain feasible with high R0 rates.
- The results support a modern reframing: N2 status may not automatically imply poor prognosis when treated with perioperative chemo-immunotherapy, provided the case is resectable and managed by a strong multidisciplinary team.
Key Takeaway Messages
- Stage III N2 resectable NSCLC: perioperative nivolumab + chemo delivers a large EFS gain (HR 0.46) and ~4× higher pCR (22% vs 5.6%).
- Multistation N2: benefit remains robust (HR 0.43; pCR 29% vs 2.7%).
- Surgical feasibility is preserved, and in N2, nivolumab was associated with fewer pneumonectomies.
- Safety is acceptable but not trivial; pneumonitis-related deaths occurred (rare but clinically important).
Conclusion
This CheckMate 77T exploratory analysis suggests that perioperative nivolumab + neoadjuvant chemotherapy can neutralize much of the historical adverse prognosis of N2 disease, even when N2 is multistation—supporting perioperative immunotherapy as a strong standard option for appropriately selected resectable stage III patients.
You Can Read All Article Here