Fresh from the podium at the ESMO Congress 2025, Dr. Matthew D. Galsky presented the extended five-year results from the landmark Phase III CheckMate 274 trial (NCT02632409), evaluating adjuvant nivolumab versus placebo in patients with high-risk muscle-invasive urothelial carcinoma (MIUC) following radical surgery.
Earlier analyses of CheckMate 274 demonstrated significant improvement in disease-free survival (DFS) with nivolumab, establishing it as a global standard of care. The updated data presented at ESMO 2025 provided deeper insights into overall survival (OS), disease-specific survival (DSS), and exploratory circulating tumor DNA (ctDNA) findings, reinforcing the long-term efficacy and safety of adjuvant immunotherapy in this challenging disease setting.
Background
Muscle-invasive urothelial carcinoma remains one of the most aggressive forms of bladder cancer, with a high risk of recurrence even after radical cystectomy or nephroureterectomy. Before the advent of immunotherapy, adjuvant treatment options were limited, and a significant proportion of patients experienced relapse within two years despite optimal surgery and, in some cases, prior neoadjuvant chemotherapy.
The CheckMate 274 trial was initiated to determine whether adjuvant PD-1 blockade with nivolumab, an immune checkpoint inhibitor targeting programmed death receptor-1, could improve outcomes compared to placebo in this high-risk population. Earlier interim and three-year analyses showed a durable improvement in disease-free survival and encouraging trends in overall survival, leading to the global adoption of nivolumab as the standard of care for adjuvant treatment in high-risk muscle-invasive urothelial carcinoma.
Methods
A total of 709 patients who had undergone radical surgery (with or without prior neoadjuvant chemotherapy) were randomized 1:1 to receive:
- Nivolumab 240 mg IV every 2 weeks for up to 1 year, or
- Placebo on the same schedule.
Eligible patients had stage pT3–pT4a or pN+ disease and were at high risk for recurrence. The co-primary endpoints were disease-free survival (DFS) in both the intent-to-treat (ITT) population and in patients with PD-L1 ≥1% tumors. Overall survival (OS) and disease-specific survival (DSS) were key secondary endpoints.
An exploratory analysis assessed ctDNA at baseline (cycle 1, day 1) using the Natera Signatera assay to evaluate its potential role as a biomarker of minimal residual disease and treatment response.
Results
After a median follow-up of 43.4 months, nivolumab continued to demonstrate long-term benefit across key efficacy endpoints:
Disease-Free Survival (DFS):
- ITT: 21.9 months with nivolumab vs 11.0 months with placebo (HR 0.74; 95% CI, 0.61–0.90)
- PD-L1 ≥1%: 55.5 vs 8.4 months (HR 0.58; 95% CI, 0.42–0.79)
Overall Survival (OS):
- ITT: 75.0 vs 50.1 months (HR 0.83; 95% CI, 0.67–1.02)
- PD-L1 ≥1%: Not reached vs 59.4 months (HR 0.63; 95% CI, 0.44–0.90)
Disease-Specific Survival (DSS):
- ITT: HR 0.79 (95% CI, 0.62–1.00)
- PD-L1 ≥1%: HR 0.57 (95% CI, 0.37–0.87)
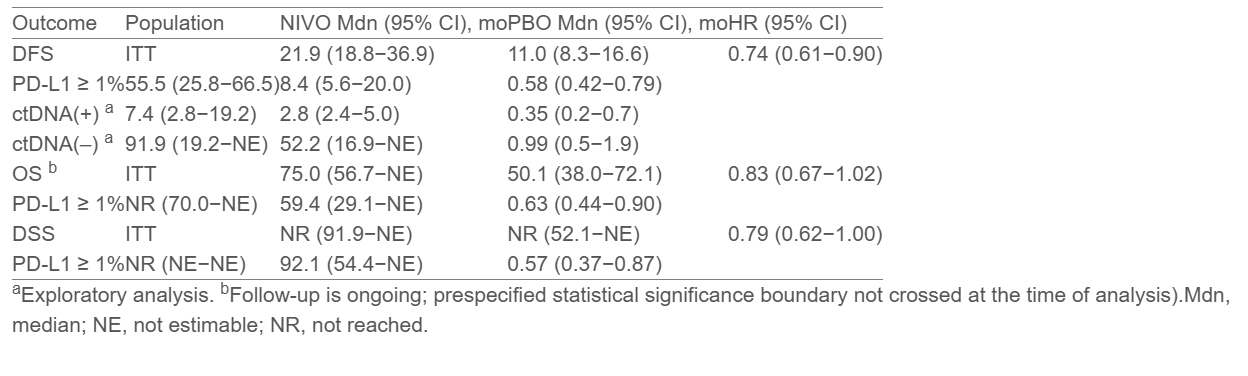
In the ctDNA analysis, 133 patients (18.8%) had evaluable samples at baseline; 40.6% were ctDNA-positive. Among ctDNA-positive patients, nivolumab markedly improved DFS compared to placebo (7.4 vs 2.8 months; HR 0.35, 95% CI, 0.20–0.70). No DFS benefit was observed in ctDNA-negative patients, suggesting ctDNA may identify individuals most likely to benefit from adjuvant immunotherapy.
No new safety signals were reported, and the safety profile of nivolumab remained consistent with prior analyses and other tumor types.
Conclusions
The five-year results from the CheckMate 274 trial confirm the durable benefit of adjuvant nivolumab in patients with high-risk muscle-invasive urothelial carcinoma after surgery. Sustained improvements in DFS, OS, and DSS were observed across both the intent-to-treat and PD-L1–positive populations.
The exploratory ctDNA analysis reinforced its potential as a biomarker for minimal residual disease and treatment response, showing greater benefit among ctDNA-positive patients. No new safety issues were identified, further supporting nivolumab as the long-term standard of care in this setting.
You can read the full abstract here.