While CAR-T cell therapies have revolutionized outcomes in hematologic malignancies, many patients still relapse. One key culprit is antigen (Ag) escape, where tumor cells downregulate or lose the expression of the target antigen—most notably CD19 in B-cell malignancies. Despite only 0.1–1% of tumor cells being antigen-negative (Ag⁻) at baseline, relapse rates due to Ag escape remain alarmingly high in both leukemia and lymphoma.
To combat this, researchers have explored mechanisms of bystander killing, where CAR-T cells eliminate not only Ag⁺ targets but also neighboring Ag⁻ tumor cells. One such mechanism involves the Fas/FasL pathway, a death receptor signaling axis that mediates T cell cytotoxicity.
Study Objective
This study investigates how Fas–FasL–mediated bystander killing can be leveraged to overcome antigen escape in CAR-T cell therapy. To explore this, the researchers modeled extreme antigen heterogeneity both in vitro and in vivo, characterized the mechanisms of Fas-dependent bystander killing, and explored pharmacologic and genetic strategies to enhance this pathway. They also evaluated clinical relevance using patient datasets and syngeneic tumor models.
Key Findings in Fas-FasL–Mediated Bystander Killing
The study uncovered multiple layers of insight into how Fas-FasL interactions can be exploited to enhance immunotherapy, especially in the context of antigen-escape in CAR-T therapy.
- FAS Expression Predicts Survival in CD19-Low Lymphoma: In patients with diffuse large B-cell lymphoma (DLBCL) treated with CD19-targeted CAR-T cells, higher tumor FAS expression correlated with improved survival—particularly in those with CD19-low tumors. This predictive association was not observed in patients receiving R-CHOP chemotherapy, highlighting that FAS expression specifically impacts CAR-T–mediated cytotoxicity, not general treatment response.
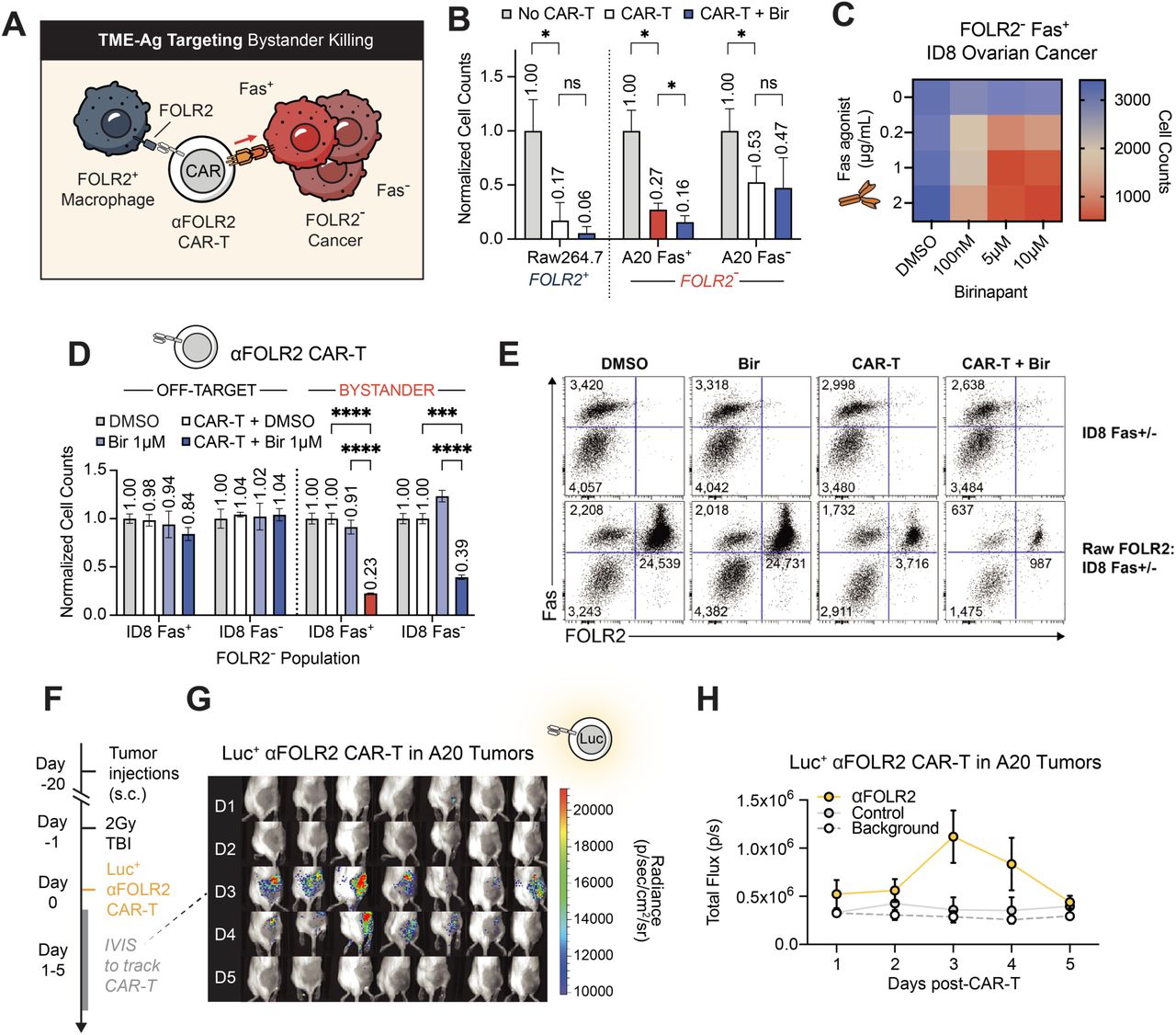
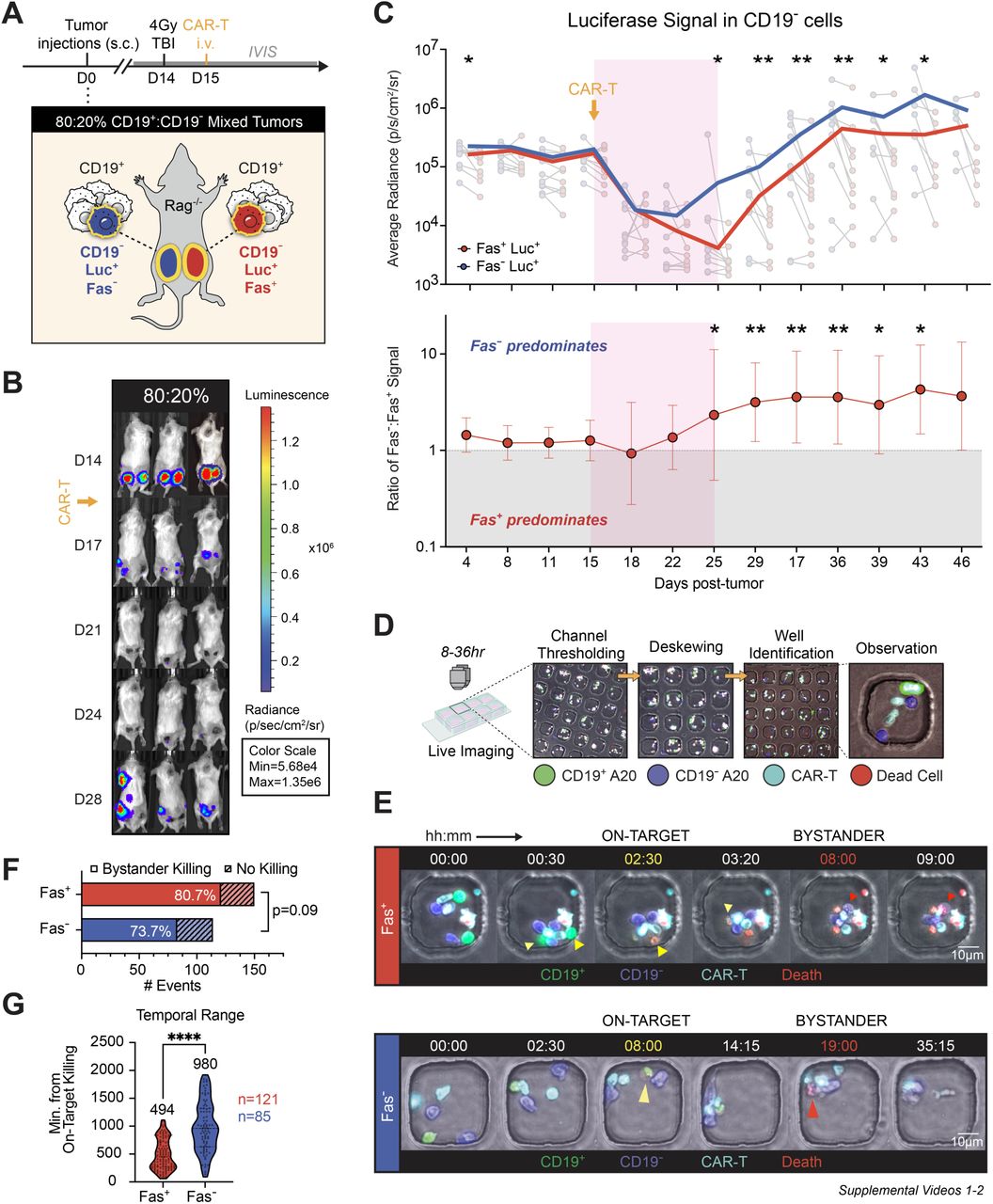
- Fas-Mediated Bystander Killing Is Real and Potent: Using both murine and human lymphoma models, the researchers demonstrated that Fas-positive, antigen-negative tumor cells could be effectively killed when co-mingled with antigen-positive targets in the presence of CAR-T cells. In contrast, Fas-negative, antigen-negative cells were largely resistant to killing, both in vitro and in vivo. Bystander killing was shown to require antigen stimulation, direct cell-cell contact, and occurred rapidly within a 10-day therapeutic window.
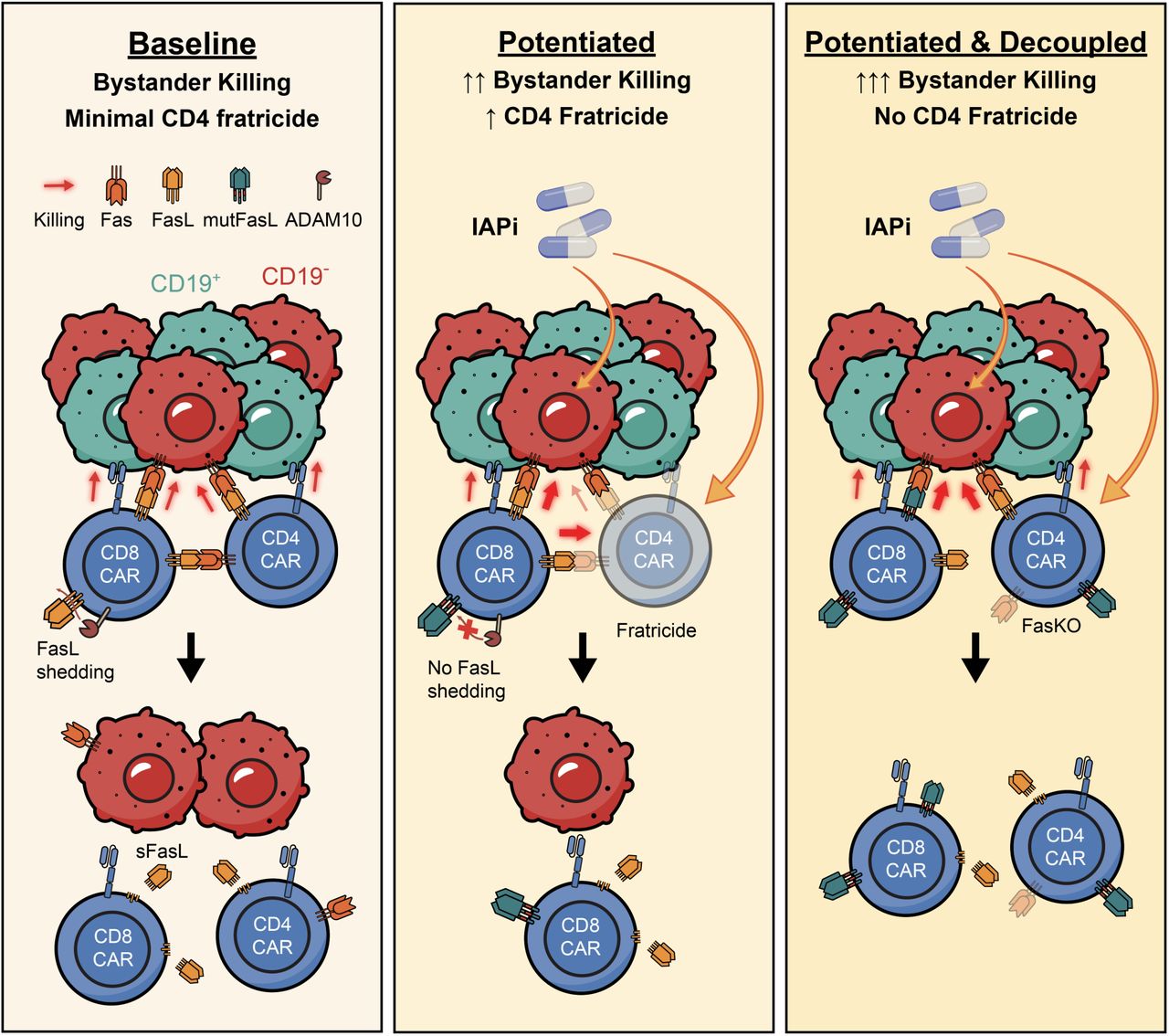
- Enhancing the Fas/FasL Axis Improves Tumor Control: To strengthen Fas-mediated bystander killing, researchers tested two main strategies aimed at boosting apoptotic signaling in Fas-positive tumor cells. First, they employed birinapant, an inhibitor of inhibitor of apoptosis proteins (IAP), which significantly enhanced apoptosis in Fas⁺ tumor cells and led to improved tumor clearance when used in combination with CAR-T cells. Second, they genetically engineered CAR-T cells to express a membrane-stabilized form of Fas ligand (mutFasL), thereby increasing the delivery of the Fas death signal to neighboring tumor cells. Both approaches proved effective in enhancing tumor cell killing; however, a major caveat emerged: each intervention also triggered fratricide among CD4⁺ CAR-T cells, a form of self-targeting that limits the overall benefit of these enhancements. This highlighted the need for additional modifications to preserve CAR-T viability while harnessing the therapeutic potential of the Fas/FasL axis.
- Fas Knockout in CAR-T Cells Solves Fratricide: To overcome this, the team genetically deleted Fas in CAR-T cells (FasKO). This modification prevented fratricide among CD4⁺ T cells and, when combined with birinapant or mutFasL, led to substantial bystander killing, achieving over 80% clearance of antigen-negative tumor cells.
- Bystander Killing Extends Beyond CAR-T to Bispecifics and Solid Tumors: Importantly, the mechanism was shown to extend beyond CAR-Ts. T cells activated by bispecific antibodies (e.g., CD20xCD3) also induced Fas-dependent bystander killing in permissive models. In Fas-resistant settings, IAP inhibition restored this function. Moreover, in solid tumor models such as ovarian cancer and melanoma, CAR-T cells targeting tumor-associated macrophages (e.g., FOLR2⁺ TAMs) were able to trigger bystander killing of neighboring antigen-negative cancer cells.
Mechanistic Insights: How Fas/FasL-Mediated Bystander Killing Works
A series of mechanistic features were identified as essential for Fas/FasL-driven bystander cytotoxicity in the tumor microenvironment:
- Fas Expression on Tumor Cells is a prerequisite, as it enables the apoptotic signaling cascade to be triggered by Fas ligand (FasL) expressed on activated CAR-T cells. Without Fas, tumor cells become resistant to this killing mechanism.
- Antigen Stimulation is required to upregulate FasL expression on CAR-T cells. This means that CAR-T cells must first recognize and engage antigen-positive tumor cells before they can execute bystander killing of nearby antigen-negative but Fas-positive cells.
- Direct Cell-Cell Contact is necessary for efficient Fas-FasL interaction. Soluble factors alone are insufficient; physical interaction between CAR-T and target cells is critical for delivering the death signal.
Membrane-Bound FasL is significantly more cytotoxic than its soluble counterpart. The anchored version provides a stronger and more localized apoptotic signal to target cells. - Inhibitor of Apoptosis Proteins (IAP) Inhibition, such as with birinapant, sensitizes tumor cells to Fas-mediated apoptosis by removing intracellular anti-apoptotic brakes, thus enhancing the effectiveness of Fas signaling.
- Fas Knockout (FasKO) in CAR-T Cells solves the problem of CD4⁺ T cell fratricide by making CAR-T cells themselves resistant to Fas-FasL–induced death, thereby improving durability and overall cytotoxic potential.
Clinical Implications
Harnessing Fas–FasL signaling presents a promising strategy to overcome antigen heterogeneity in tumors. By combining CAR‑T therapy with complementary approaches—such as IAP inhibitors to sensitize tumor cells, stabilization of FasL to enhance its cytotoxic effect, and Fas knockout to protect CAR‑T cells from fratricide—it may be possible to prevent antigen escape and achieve deeper, more durable remissions.
Moreover, CAR‑T cells designed to target components of the tumor microenvironment rather than tumor antigens directly could be repurposed to induce potent bystander killing. This capability expands immunotherapy beyond traditional antigen-positive targets, offering a new avenue for treating antigen‑poor or highly heterogeneous solid tumors where current therapies are limited.
Future Directions
- Temporal limitations of FasL expression warrant exploration of programmable FasL systems.
- Combinatorial platforms, such as logic-gated CARs or bispecific secreting CARs, may extend the therapeutic window.
- Fas-independent mechanisms, like IFNγ/TNFα-driven bystander killing, remain important and should be integrated.

You Can Read The All Article Here.