The European Commission has approved lisocabtagene maraleucel (Breyanzi), a CD19-directed CAR T-cell therapy, for adults with relapsed or refractory mantle cell lymphoma (MCL) after at least two prior lines of systemic therapy, including a BTK inhibitor. This decision applies to all EU member states and EEA countries (Iceland, Norway, Liechtenstein).
Why this matters
Mantle cell lymphoma is a rare, aggressive B-cell non-Hodgkin lymphoma, accounting for ~3% of NHL cases, typically diagnosed in older adults and characterized by frequent relapse and treatment resistance.
After BTK inhibitors fail, options are limited and prognosis is poor, so an additional CAR T option in this space addresses a clear high-risk population.
Key efficacy data – TRANSCEND NHL 001 (MCL cohort)
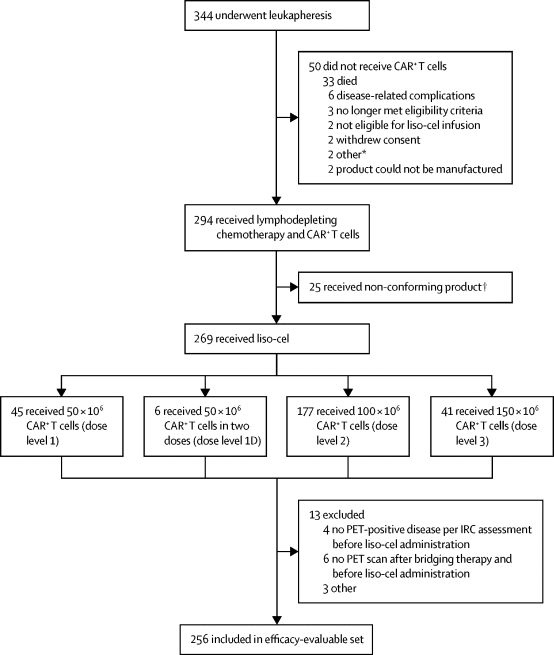
Approval is based on the MCL cohort of the TRANSCEND NHL 001 trial, which enrolled adults with r/r MCL after ≥2 prior lines, including a BTK inhibitor. Patients received a single Breyanzi infusion after lymphodepleting chemotherapy.
Responses
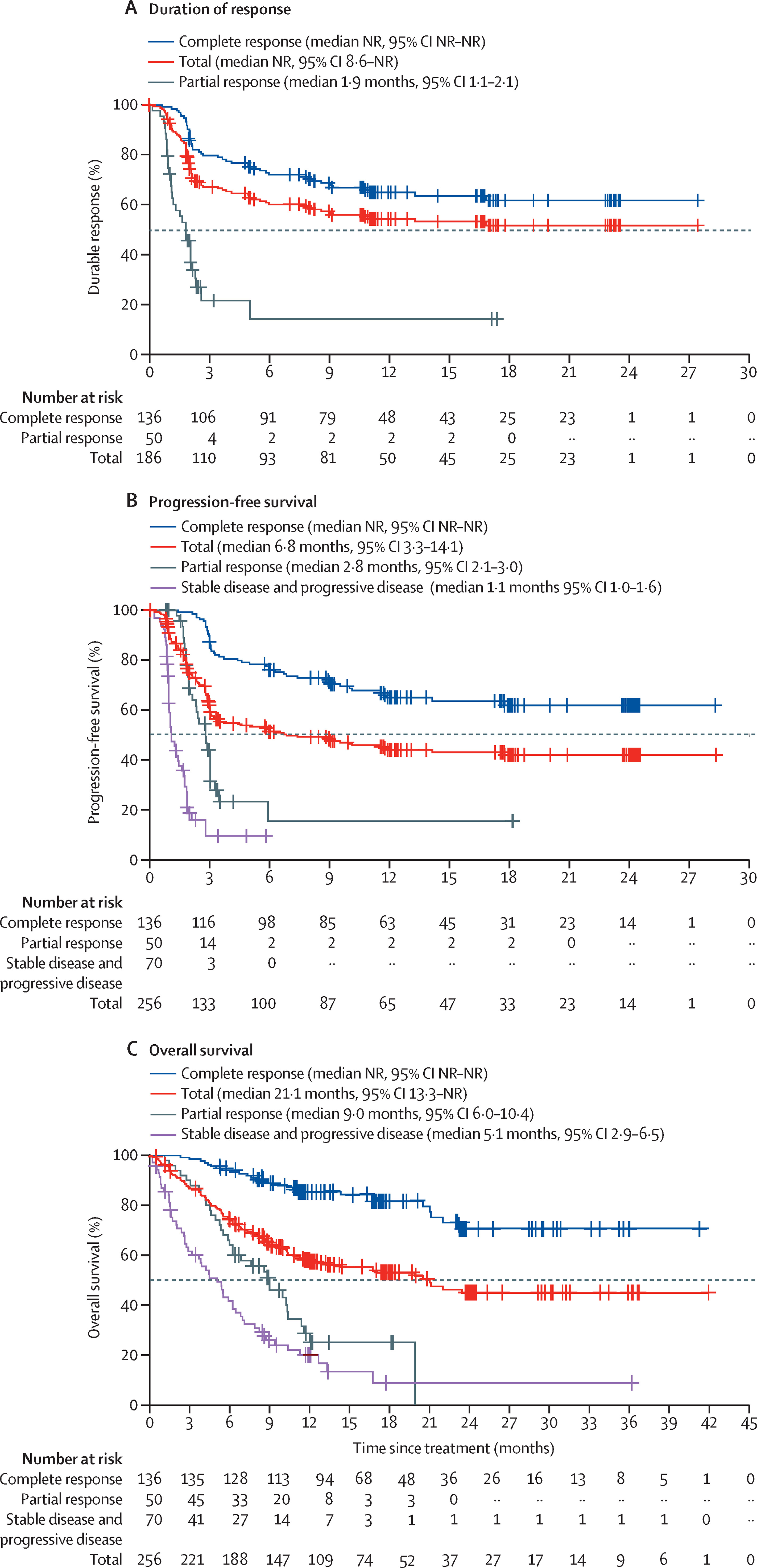
- Overall response rate (ORR): 82.7% (95% CI 72.7–90.2)
- Complete response (CR) rate: 71.6% (95% CI 60.5–81.1)
- Median time to first response (CR or PR): 0.95 months (range 0.7–3.0)
- At 24 months, 41.2% of patients remained in response, indicating durable benefit for a substantial proportion of this heavily pretreated group.
These results are notable given how refractory BTK-exposed MCL typically behaves and position Breyanzi as a high-depth, relatively rapid-onset option.
Safety profile
The safety findings in MCL were consistent with the established CAR T safety profile seen with Breyanzi in other large B-cell lymphomas:
Cytokine release syndrome (CRS):
- Any grade: 61%
- Grade 3–4: 1%
- Median time to onset: 4 days (range 1–10 days)
Neurologic toxicities (ICANS and others):
- Any grade: 31%
- Grade 3–4: 9%
- Median time to onset: 8 days (range 1–25 days)
Most CRS and neuro events occurred within the first 2 weeks post-infusion and were generally reversible with appropriate management, supporting current practice of early, intensive monitoring in CAR T centers.
Clinical takeaways
- Target population: Adults with r/r MCL after ≥2 lines including a BTKi — a group with high unmet need and limited curative options.
- Efficacy: High ORR (82.7%) and CR rate (71.6%) with ~50% of patients still in response at 2 years suggest meaningful long-term disease control for many.
- Safety: Predictable CAR T-type toxicities (CRS, neurotoxicity) with relatively low rates of grade ≥3 CRS and manageable timing, supporting use in experienced centers.
- Practice impact: Breyanzi adds another CD19 CAR T option for post-BTKi MCL in Europe and may shift treatment algorithms toward earlier referral for cellular therapy in eligible patients.