Regional nodal irradiation (RNI) is standard for breast cancer patients with pathologically positive axillary lymph nodes after surgery, reducing recurrence. However, its benefit in patients who have a pathologic complete response (ypN0) after neoadjuvant chemotherapy is unclear. The NRG‑NSABP B‑51/RTOG 1304 trial investigates whether omitting RNI is non-inferior in this specific cohort
Title:Omitting Regional Nodal Irradiation after Response to Neoadjuvant Chemotherapy
Authors: Eleftherios P. Mamounas, M.D., Hanna Bandos, Ph.D., Julia R. White, M.D., Thomas B. Julian, M.D., Atif J. Khan, M.D., Simona F. Shaitelman, M.D., Mylin A. Torres, M.D., Frank A. Vicini, M.D., Patricia A. Ganz, M.D., Susan A. McCloskey, M.D., Peter C. Lucas, M.D., Ph.D., Nilendu Gupta, Ph.D., X. Allen Li, Ph.D., Beryl McCormick, M.D., Benjamin Smith, M.D., Rahul D. Tendulkar, M.D., Vivek S. Kavadi, M.D., Koji Matsumoto, M.D., Samantha Andrews Seaward, M.D., William J. Irvin, Jr., M.D., Jolinta Y. Lin, M.D., Robert W. Mutter, M.D. Thierry M. Muanza, M.Sc., Jannifer Stromberg, M.D., Reshma Jagsi, M.D., Ph.D., Anna C. Weiss, M.D., Walter J. Curran, Jr., M.D., and Norman Wolmark, M.D.
Published in NEJM, June 2025
Study Design and Methods
The NRG‑NSABP B‑51/RTOG 1304 trial was conducted at multiple academic and community oncology centers across the United States and Canada under the auspices of the NRG Oncology cooperative group. The study followed international Good Clinical Practice guidelines, and institutional review board approval was obtained at each participating site. All patients provided written informed consent.
Eligibility criteria included women aged ≥18 years with newly diagnosed, biopsy-proven clinical stage T1–T3, N1, M0 breast cancer, confirmed by core needle biopsy of the primary tumor and axillary lymph node. Patients must have completed neoadjuvant chemotherapy, followed by either lumpectomy or mastectomy with axillary surgical staging. Only patients with pathologic node-negative status (ypN0) after surgery, as confirmed by central pathology review or institutional pathology reports, were eligible for randomization.
Key exclusion criteria included inflammatory breast cancer, evidence of distant metastases (M1), prior malignancy likely to interfere with study outcomes, previous ipsilateral thoracic radiation, or any contraindication to radiation therapy.
Following surgery and pathologic assessment, eligible patients were randomized in a 1:1 ratio to one of two treatment arms:
- Regional Nodal Irradiation (RNI) Arm: Received adjuvant radiation to the breast or chest wall (depending on type of surgery), plus nodal irradiation to the supraclavicular, infraclavicular, internal mammary, and high axillary lymph nodes.
- No-RNI Arm: Received radiation to the breast or chest wall alone, without nodal irradiation.
Randomization was conducted using a permuted block design, stratified by:
- Type of breast surgery (lumpectomy vs mastectomy).
- Hormone receptor status (positive vs negative).
- HER2 status (positive vs negative).
- Use of adjuvant systemic therapy (yes vs no).
- Pathologic complete response (pCR) in the breast (yes vs no).
Systemic therapy was administered according to standard institutional practice. Patients with HER2-positive tumors received trastuzumab-based anti-HER2 therapy. Hormone receptor–positive tumors were treated with endocrine therapy for at least five years. Postmastectomy chest wall radiation or whole-breast radiation was required in both arms as appropriate. Boost radiation was permitted per local practice.
The primary endpoint was invasive breast cancer recurrence–free interval (IBCRFI), defined as time from randomization to the first occurrence of:
- Ipsilateral invasive breast tumor recurrence
- Locoregional invasive recurrence
- Distant recurrence
- Breast cancer–specific death
Secondary endpoints included
- Locoregional recurrence
- Distant recurrence
- Disease-free survival
- Overall survival
- Radiation-related adverse events and toxicities.
Adverse events were graded using the NCI Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
The trial aimed to accrue 1,636 patients, powered to detect a hazard ratio of 0.75 with 80% power, using an alpha level of 0.05 (one-sided), assuming a 5-year IBCRFI rate of 92% in the no-RNI group and a 3.2% absolute difference deemed clinically relevant.
Patients were followed with clinical assessments, imaging, and toxicity evaluations at regular intervals according to protocol guidelines, with a planned follow-up duration of at least 10 years. The results reported in this analysis reflect an interim analysis at a median follow-up of 59.5 months.
Results
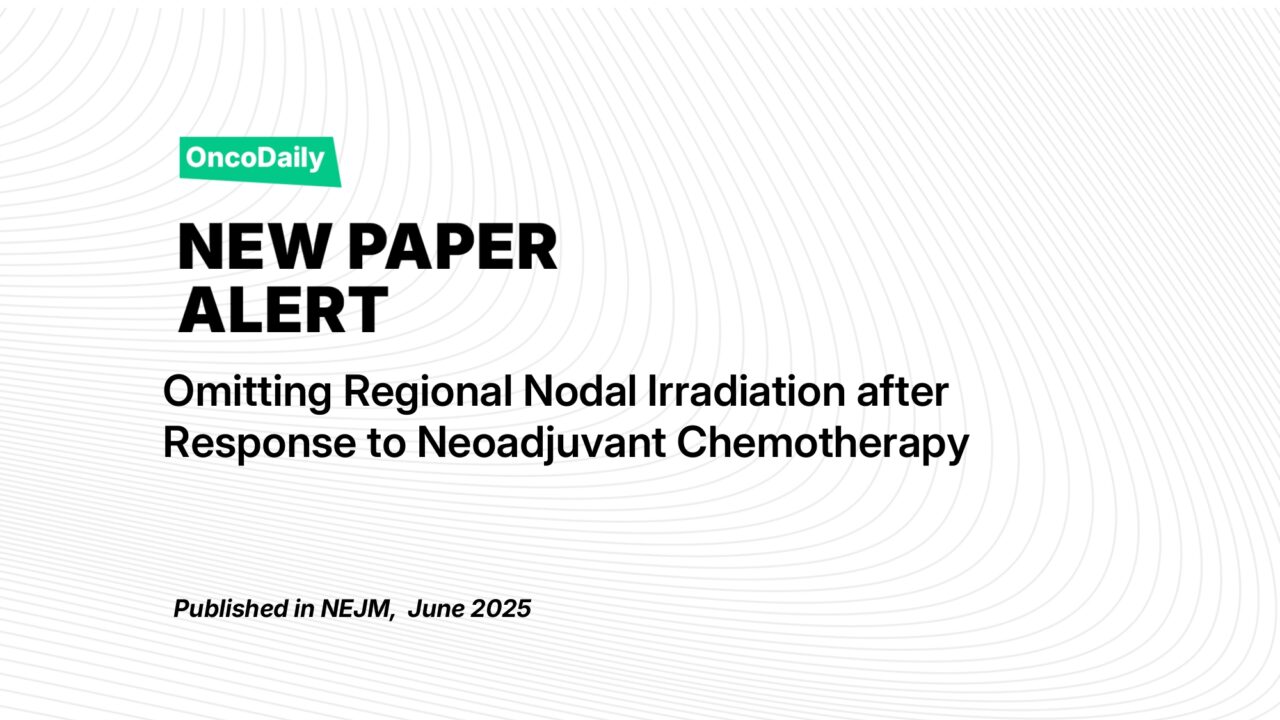
The NRG‑NSABP B‑51/RTOG 1304 trial demonstrates that in patients initially presenting with node-positive breast cancer who achieve pathologic node-negative status post-neoadjuvant chemotherapy, adding regional nodal irradiation does not significantly improve invasive recurrence-free survival or other oncologic outcomes up to five years These results support omitting RNI in appropriately selected ypN0 patients to spare them potential side effects, while achieving excellent disease control. Clinicians should consider pathologic response to guide tailored radiation therapy, although ongoing surveillance will confirm long-term durability.
- Primary outcome: 109 IBCRFI events occurred (50 in RNI arm; 59 in no-RNI arm).
- Hazard ratio (HR) = 0.88 (95% CI: 0.60–1.28; P = 0.51).
- 5‑year IBCRFI: 92.7% with RNI vs. 91.8% without RNI bioengineer.org+2pubmed.ncbi.nlm.nih.gov+2eurekalert.org+2bioengineer.org.
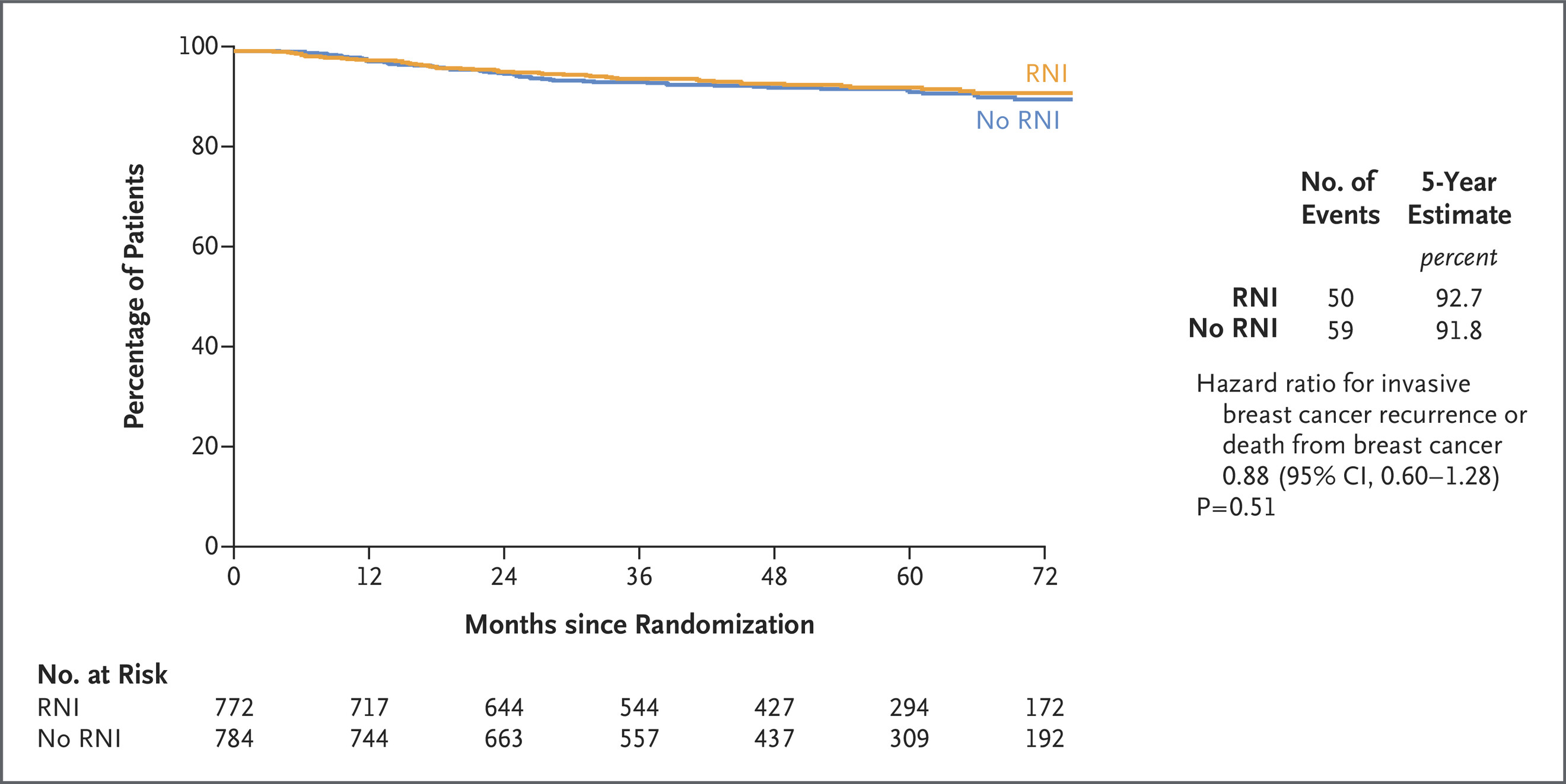
Secondary outcomes: No significant differences observed in locoregional recurrence, distant recurrence, disease‑free survival, or overall survival between arms nejm.org+15pubmed.ncbi.nlm.nih.gov+15eurekalert.org+15.
Safety: Grade 4 adverse events were rare—0.5% in the RNI group vs. 0.1% in the no‑RNI group. No treatment-related deaths or unexpected toxicity were reported bioengineer.org+2pubmed.ncbi.nlm.nih.gov+2eurekalert.org+2.
Key Findings
- Omission of RNI after achieving ypN0 status post‑neoadjuvant chemotherapy did not compromise invasive recurrence-free survival (HR 0.88; P = 0.51).
- High five-year IBCRFI rates (>91%) were observed in both groups, supporting excellent outcomes even without nodal irradiation.
- No benefit was found in secondary efficacy measures or overall survival with added RNI.
- Low-grade adverse events suggest good tolerability across both arms.
Key Takeaway Messages
- Pathologic nodal clearance (ypN0) after neoadjuvant therapy may identify a low-risk group that does not benefit from additional regional nodal irradiation.
- Radiation omission in this setting can reduce unnecessary treatment burden without affecting oncologic outcomes.
- Treatment can be individualized based on response; ypN0 status serves as a strong biomarker to guide radiation decisions.
- Longer-term follow-up is still necessary to monitor durable disease control and late toxicity.
You can read the full article here
Written by Sona Karamyan, MD