France has expanded national compassionate access to the investigational immunotherapy combination botensilimab (BOT) plus balstilimab (BAL), extending fully reimbursed hospital-based use beyond refractory microsatellite-stable (MSS) colorectal cancer to include selected ovarian cancers and soft-tissue sarcomas.
On January 12, 2026, Agenus Inc. announced that France’s National Agency for Medicines and Health Products Safety (ANSM) approved an updated national protocol under the Autorisation d’Accès Compassionnel (AAC) framework. The revised authorization broadens eligibility for BOT/BAL to additional solid tumor indications with substantial unmet medical need after exhaustion of standard therapies.
Under the AAC program, treatment is fully reimbursed by France’s national health system (Assurance Maladie), with standardized hospital oversight and structured real-world data collection.
Understanding Botensilimab and Balstilimab
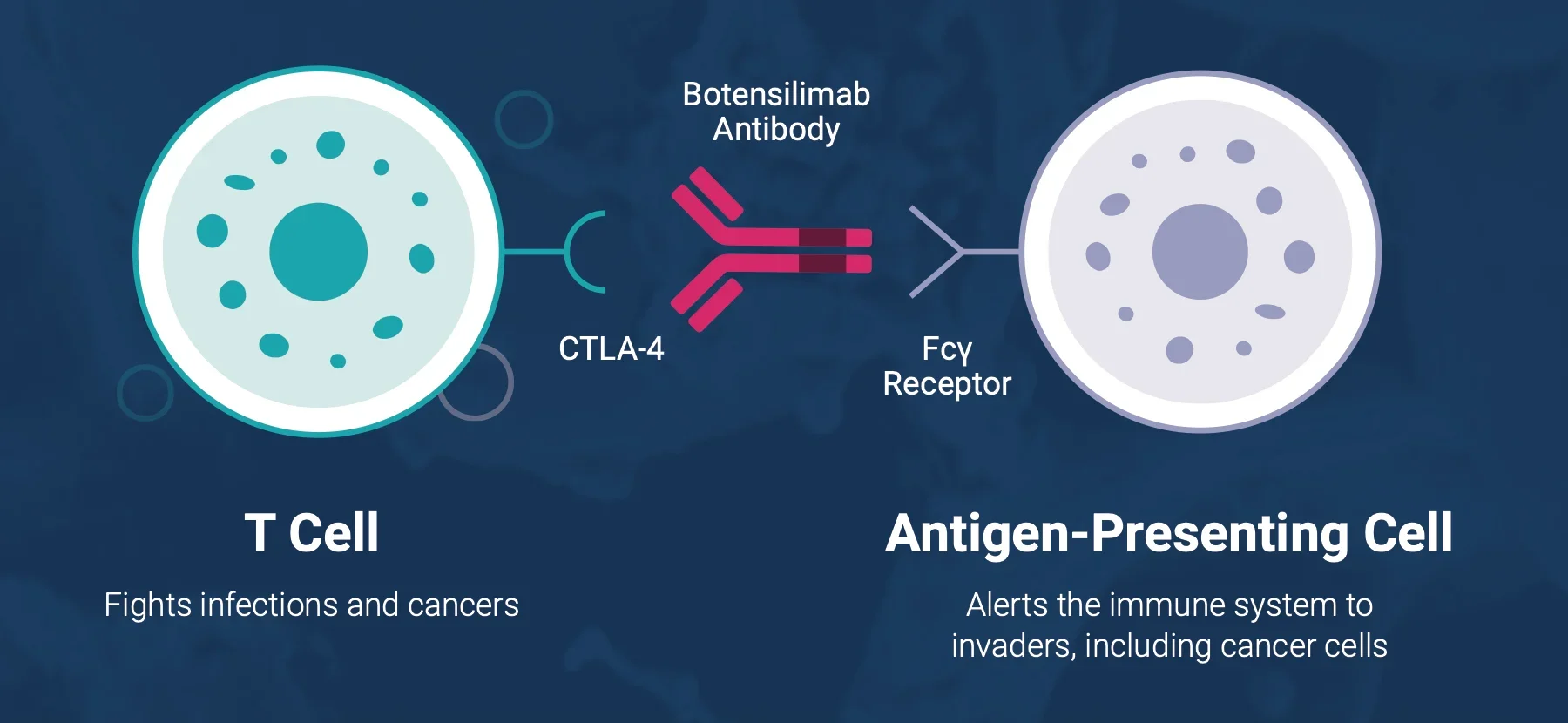
Botensilimab (BOT) is a human, Fc-enhanced anti-CTLA-4 antibody engineered to stimulate both innate and adaptive immunity. Its multifunctional design enables activity in “cold” tumors by:
- Priming and activating T cells
- Reducing intratumoral regulatory T cells
- Engaging myeloid compartments
- Promoting durable immune memory
Balstilimab (BAL) is a fully human IgG4 anti-PD-1 antibody that blocks PD-1 interaction with PD-L1 and PD-L2, releasing inhibitory signaling on activated T cells.
Together, BOT and BAL are designed to deliver synergistic immune activation, broadening the reach of checkpoint immunotherapy across resistant tumor microenvironments.
Botensilimab/Balstilimab
What the Updated AAC Authorization Covers
The expanded AAC protocol now authorizes reimbursed access to BOT/BAL for eligible adult patients with:
- MSS metastatic colorectal cancer without active liver metastases, following progression on standard therapies
- Platinum-refractory or platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer, after approved options have been exhausted
- Advanced or metastatic soft-tissue sarcomas, including multiple high-grade histologies, after failure of standard treatments
This expansion builds on France’s earlier AAC authorization in MSS colorectal cancer and represents a multi-tumor national early-access framework for a single investigational immunotherapy combination—an uncommon level of coordinated national authorization.

Botensilimab + Balstilimab in Platinum-Resistant Ovarian Cancer
How France’s AAC Framework Enables Early Access
France’s AAC pathway allows hospital-based access to investigational therapies for patients with serious or life-threatening diseases who lack appropriate therapeutic alternatives. Treatment is governed by a nationally standardized protocol, defining eligibility criteria, administration, safety monitoring, and outcome reporting under ANSM oversight.
For BOT/BAL, reimbursement is structured as a single, course-based payment per patient, covering the full treatment course once authorization is granted. Hospitals receive full reimbursement outside the standard diagnosis-related group system (“en sus du GHS”), ensuring access without financial burden to patients or institutions.
Scientific Rationale and Emerging Clinical Evidence
BOT/BAL is a chemotherapy- and radiation-free immunotherapy combination designed to extend immune checkpoint activity into traditionally immunotherapy-resistant tumors.
Across clinical studies conducted to date, BOT/BAL has demonstrated antitumor activity in heavily pretreated populations, including tumor types with historically limited responsiveness to standard PD-1–based approaches. Signals of durable benefit have been observed in multiple solid tumors, supporting exploration beyond colorectal cancer.
The AAC expansion reflects growing confidence in the combination’s biologic rationale, emerging efficacy signals, and manageable safety profile, while additional prospective and real-world data continue to mature.
You Can Watch More on OncoDaily Youtube TV
BOT/BAL Remains Investigational
Botensilimab plus balstilimab is not approved for commercial use in France or elsewhere. Access is limited to:
- Clinical trials, including the ongoing Phase 3 BATTMAN study in refractory MSS colorectal cancer
- Regulatory-authorized early-access programs, such as France’s AAC framework
Outside France, access may be available through named-patient programs depending on local regulations and reimbursement policies.

Botensilimab/Balstilimab
Read about BOT/BAL Advancements, BATTMAN Trial, FDA Approval Challenges and Key Highlights from Agenus Stakeholder Briefing on OncoDaily.


