Immune checkpoint inhibition has transformed the first-line treatment of advanced non-small cell lung cancer (NSCLC), particularly in tumors with high PD-L1 expression (TPS ≥ 50%), where single-agent PD-1/PD-L1 blockade became a global standard.Yet even in this biomarker-enriched population, primary resistance remains common, and a substantial proportion of patients either fail to respond or experience early progression. This clinical gap has driven interest in dual-checkpoint strategies that simultaneously target complementary immunosuppressive pathways within the tumor microenvironment.
One of the most biologically compelling partners for PD-1 blockade is TIGIT, an inhibitory receptor expressed on T cells and natural killer (NK) cells that suppresses antitumor immunity through interaction with CD155. Preclinical and early clinical data suggest that combined TIGIT and PD-1 inhibition may produce deeper and more durable immune activation than PD-1 blockade alone.
Domvanalimab represents a distinctive approach within this class. Unlike several earlier anti-TIGIT antibodies, it is engineered with a silent Fc domain, designed to avoid depletion of TIGIT-expressing immune cells, preserve immune homeostasis, and potentially reduce immune-related toxicity while maintaining antitumor efficacy.
Study Design and Methods
ARC-10 study was a global, randomized, open-label phase 2 study evaluating first-line therapy in patients with:
- Stage IIIB–IV NSCLC
- PD-L1 TPS ≥ 50% by 22C3 assay
- No actionable driver mutations
- ECOG performance status 0–1
Patients were randomized 2 : 2 : 1 to receive:
- Domvanalimab + zimberelimab (D+Z) every 3 weeks
- Zimberelimab monotherapy (Z)
- Platinum-doublet chemotherapy
- The primary endpoint was progression-free survival (PFS).
Key secondary endpoints included:
- Overall survival (OS)
- Objective response rate (ORR)
- Safety and tolerability
Enrollment ended early due to evolving treatment standards, meaning results should be interpreted as signal-seeking rather than definitive.
Results
Patient Population
In ARC-10 Part 1, a total of 98 patients were randomized, of whom 95 ultimately received study treatment. At the time of analysis, the median follow-up reached approximately 24.5 months, allowing for a meaningful early assessment of durability signals despite the modest cohort size.
Baseline characteristics were broadly representative of a typical advanced NSCLC population treated in the first-line setting. Most patients had stage IV disease, the majority were current or former smokers, and all tumors demonstrated high PD-L1 expression, consistent with the biomarker-selected design of the study. Importantly, clinically relevant subgroups—including patients with brain and liver metastases—were also meaningfully represented, supporting the real-world relevance of the dataset.
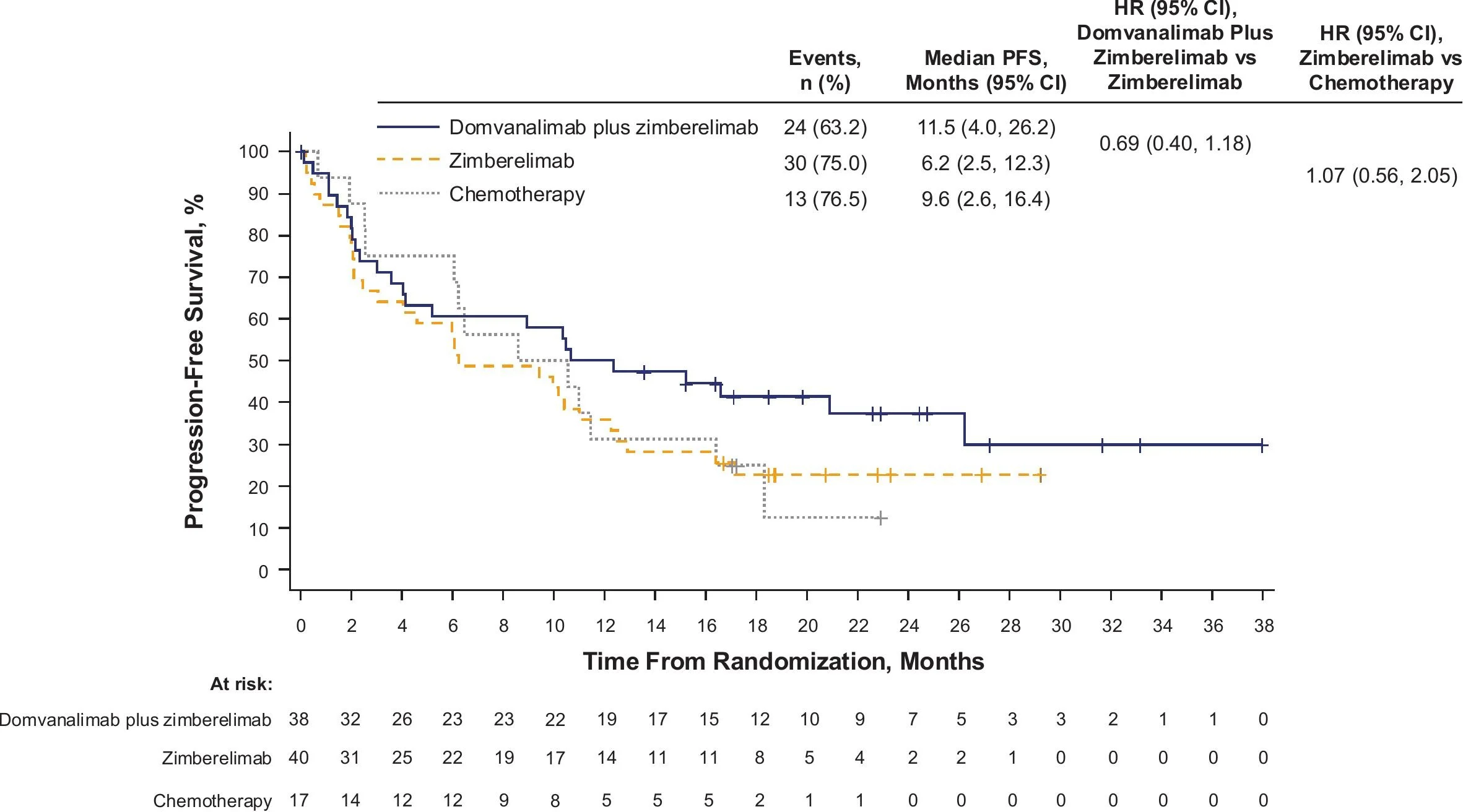
Progression-Free Survival
Dual checkpoint inhibition with domvanalimab plus zimberelimab (D+Z) produced a clear improvement in disease control compared with PD-1 blockade alone. Median progression-free survival reached 11.5 months with the combination, compared with 6.2 months for zimberelimab monotherapy and 9.6 months for platinum-doublet chemotherapy.
The hazard ratio of 0.69 for D+Z versus Z indicates a clinically meaningful reduction in the risk of progression or death, providing the strongest early efficacy signal supporting simultaneous TIGIT and PD-1 inhibition.
From a biological standpoint, this degree of separation in PFS aligns with the hypothesis that parallel checkpoint blockade may overcome primary resistance mechanisms that limit PD-1 monotherapy.
Overall Survival
Although survival data remain immature, early trends favor the dual-checkpoint strategy. Median overall survival was not yet reached in the D+Z arm, whereas it measured 24.4 months with zimberelimab alone and 11.9 months with chemotherapy.
The hazard ratio of 0.64 for D+Z versus Z, together with one-year survival rates approaching 68%, suggests a potentially meaningful long-term advantage. By comparison, approximately 57% of patients receiving zimberelimaband 50% receiving chemotherapy were alive at one year.
While these findings require confirmation in larger phase 3 trials, the non-reached median OS in the combination arm represents the most clinically provocative signal emerging from ARC-10 Part 1.
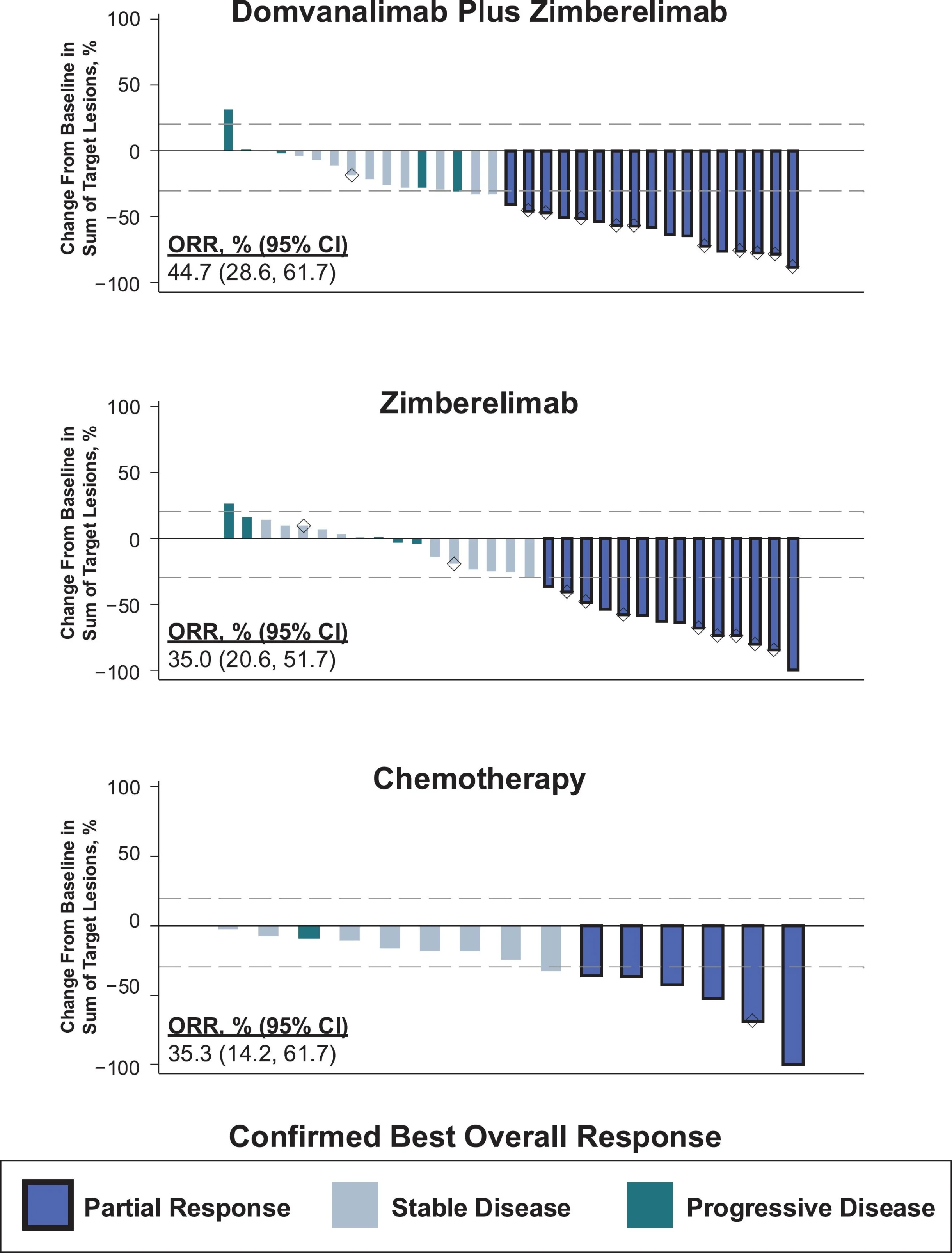
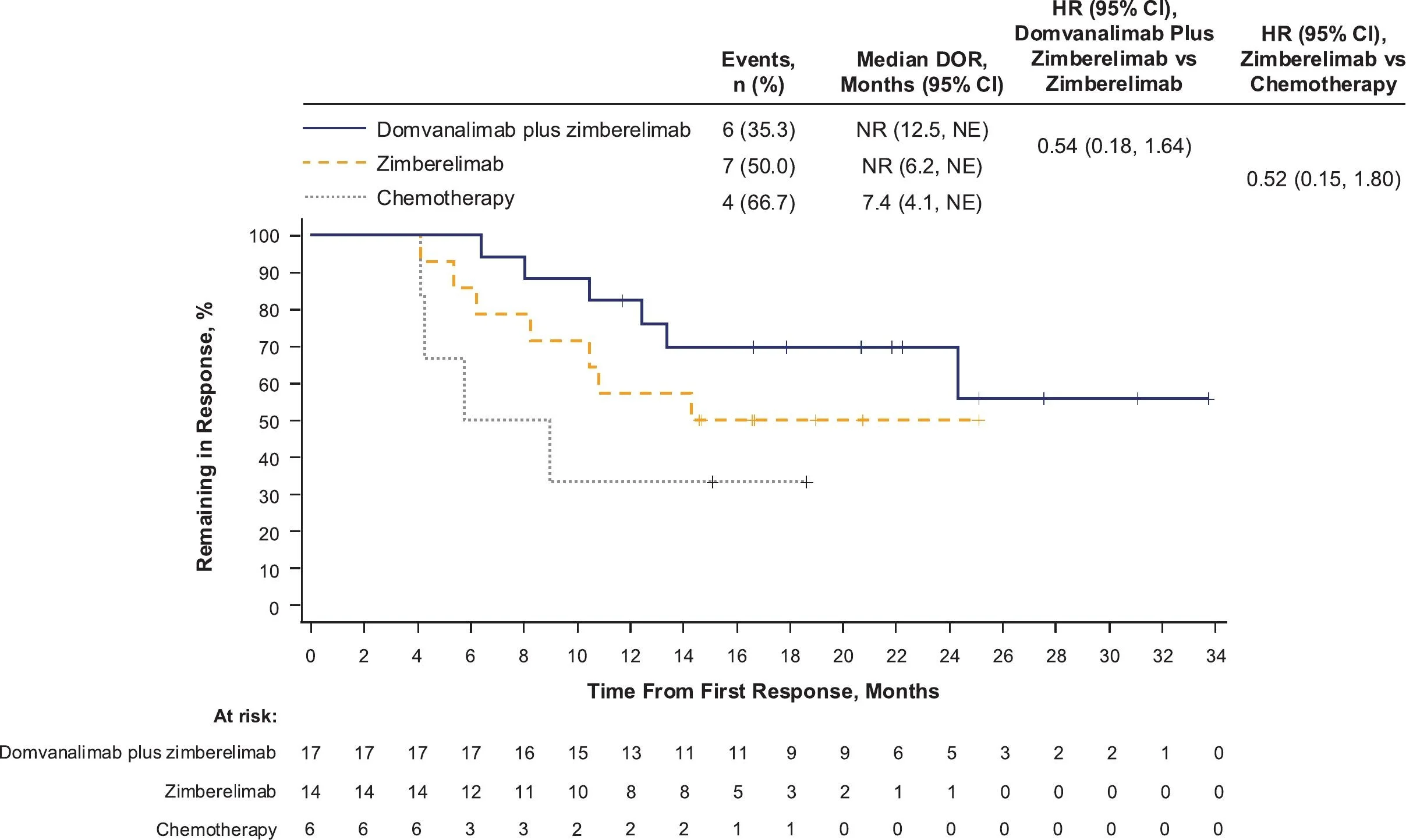
Objective Response Rate and Durability
Confirmed objective response rates were numerically higher with dual immunotherapy (44.7%) than with zimberelimab alone (35.0%) or chemotherapy (35.3%).
However, the more meaningful distinction appears to lie not in the magnitude of tumor shrinkage, but in the durability of disease control. Responses in the immunotherapy-containing arms persisted longer, reinforcing a pattern commonly observed across effective checkpoint-based combinations—where long-term immune control, rather than dramatic initial response rates, ultimately drives clinical benefit.
Safety and Tolerability
Severe toxicity
Grade ≥ 3 treatment-related adverse events:
- 21% with D+Z
- 15% with Z
- 47% with chemotherapy
Immune-mediated toxicity
- Similar frequency between D+Z and Z
- Mostly low-grade endocrine events
- Very low infusion-reaction rates
Interpretation: The addition of domvanalimab did not meaningfully worsen immune toxicity, supporting the Fc-silent design hypothesis.
Key Takeaway Messages
- Dual TIGIT + PD-1 inhibition (D+Z) improved outcomes versus PD-1 alone and chemotherapy in advanced NSCLC.
- PFS: 11.5 vs 6.2 vs 9.6 months (HR 0.69).
OS: Not reached vs 24.4 vs 11.9 months (HR 0.64).
ORR: 44.7% with greater durability of response. - Benefit appears driven by sustained disease control and survival, rather than large increases in initial tumor shrinkage—consistent with effective immunotherapy biology.
- Findings provide proof-of-concept for TIGIT + PD-1 synergy, but remain preliminary and require phase 3 confirmation before practice change.
You Can Read All Article Here