What was the Background and Rationale for the ANCHOR Trial?
Anti-VEGF antibodies combined with chemotherapy are the first-line standard for unresectable metastatic colorectal cancer (mCRC). However, no randomized trials had previously evaluated oral VEGFR-TKIs (e.g., anlotinib) combined with chemotherapy in this setting. The ANCHOR trial aimed to assess the non-inferiority of anlotinib plus CapeOX compared to bevacizumab plus CapeOX in RAS/BRAF wild-type mCRC.
How was the Study Designed and Who were the Patients?
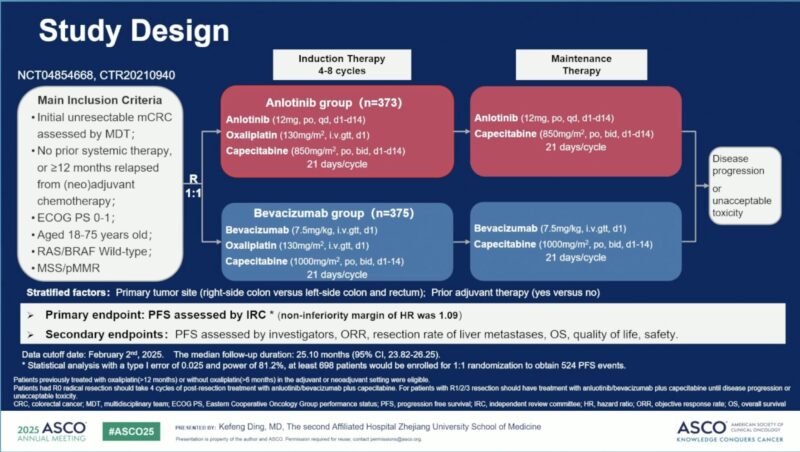
In this Chinese multicenter, randomized, non-inferiority, phase 3 trial, treatment-naïve patients with RAS/BRAF wild-type mCRC and unresectable metastases were randomized 1:1 to:
- Anlotinib (12 mg orally, days 1–14) + CapeOX (oxaliplatin 130 mg/m² IV + capecitabine 850 mg/m² BID, days 1–14), or
- Bevacizumab (7.5 mg/kg IV, day 1) + CapeOX (oxaliplatin 130 mg/m² IV + capecitabine 1000 mg/m² BID, days 1–14).
Stratification factors included tumor location (right/left) and prior adjuvant chemotherapy. Maintenance therapy continued until progression or intolerance. The primary endpoint was IRC-assessed progression-free survival (PFS), with a non-inferiority margin of HR ≤1.09. Secondary endpoints included ORR, DCR, OS, and safety.
What were the Key Results of the Trial?
Between May 2021 and August 2023, 748 patients were randomized (373 anlotinib, 375 bevacizumab). Median age was 59 years, and 30.4% were female. At a median follow-up of 25.1 months:
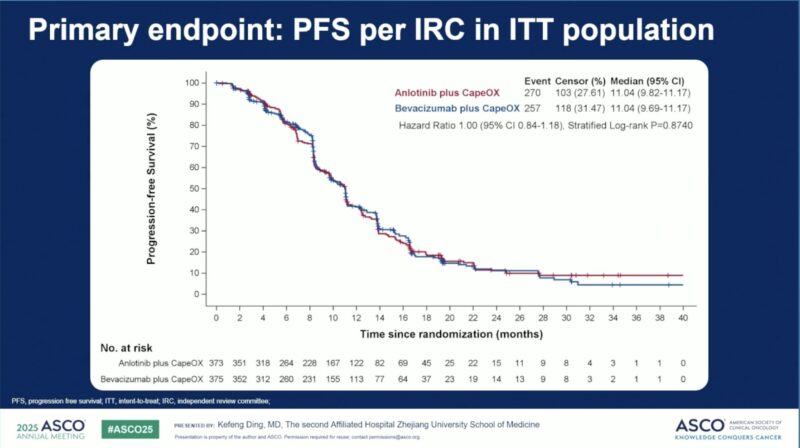
- PFS: Median PFS was 11.04 months in both arms (HR 1.00; 95% CI 0.84–1.18), meeting non-inferiority.
Response Rates:
ORR: 61.93% (anlotinib) vs 62.13% (bevacizumab).
DCR: 92.76% vs 93.07%.
Safety:
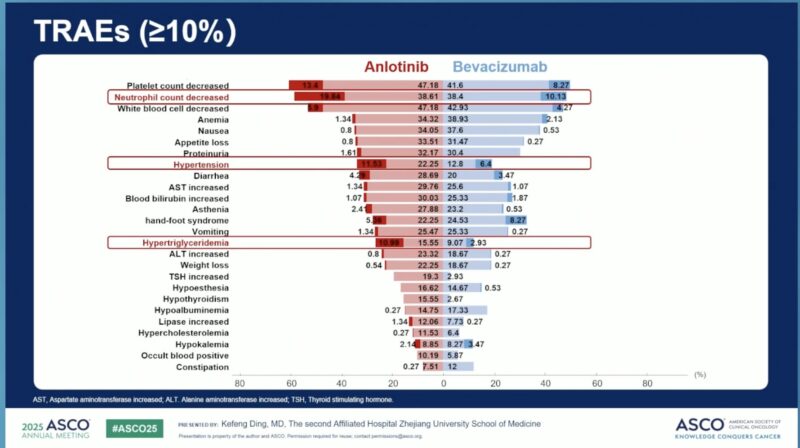
- Grade ≥3 TEAEs: 74.0% (anlotinib) vs 59.2% (bevacizumab).
- Common TEAEs included hypertension, hand-foot syndrome, and diarrhea.
- Treatment discontinuation due to TEAEs: 8.0% vs 9.1%.
- TEAE-related deaths: 4.3% vs 4.5%.
Outcome Anlotinib + CapeOX (n=373) Bevacizumab + CapeOX (n=375) HR/OR (95% CI)
Median PFS (IRC) 11.04 months 11.04 months 1.00 (0.84–1.18)
ORR 61.93% 62.13% —
DCR 92.76% 93.07% —
Liver Metastasis Resection 3.75% 2.93%
What Experts Say About the ANCHOR Trial Results?
Anlotinib vs Beva added to CTx for RAS/BRAF WT, unresectable mCRC, #ASCO25 ANCHOR Phs III,ORR 61 vs 61%, mPFS 7 vs 6.9 mo, TRAE ≥3 64% vs 44%, not sure, whether & how this trial could change mCRC treatment in the future.
From Arndt Vogel’s X
What Conclusions were Drawn from the Study?
Anlotinib combined with CapeOX demonstrated non-inferior progression-free survival compared to bevacizumab plus CapeOX in RAS/BRAF wild-type mCRC. While the safety profiles differed, both regimens showed comparable efficacy, providing a new oral VEGFR-TKI-based option for first-line treatment of unresectable mCRC.
Authors
Ke-Feng Ding, Yue Liu, Yanqiao Zhang, Rongbo Lin, Xiaobing Chen, Junye Wang, Mudan Yang, Xiujuan Qu, Yunfeng Li, Jiayi Li, Weisheng Zhang, Yong Mao, Ziwei Wang, Zhenyang Liu, Ying Cheng, Jian Lei, Ye Xu, Yi Jiang, Zhanyu Pan, Liangjun Zhu, ANCHOR Trial’s Group.
You Can Also Read Immunotherapy for Rectal Cancer: Types, Success Rate, Side Effects and More by Oncodaily
Organizations
Department of Colorectal Surgery and Oncology, Key Laboratory of Cancer Prevention and Intervention, Ministry of Education, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China; Gastroenterology, Harbin Medical University Cancer Hospital, Harbin, China; Gastrointestinal Medical Oncology, Fujian Cancer Hospital, Fuzhou, China; Henan Cancer Hospital, Zhengzhou, China; Department of Oncology, The Affiliated Hospital of Jining Medical College, Jining, China; Department of Gastroenterology, Shanxi Cancer Hospital, Taiyuan, China; The First Hospital of China Medical University, Shenyang, China; Yunnan Cancer Hospital, Kunming, Yunnan, China; The First Affiliated Hospital of Xiamen University, Xiamen,
China; Gansu Provincial Hospital, Lanzhou, China; Affiliated Hospital of Jiangnan University, Wuxi, China; The First Affiliated Hospital of Chongqing Medical University, Chongqing, China; Hunan Cancer Hospital, Changsha, China; Department of Thoracic Oncology, Jilin Cancer Hospital, Changchun, China; The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China; Department of Colorectal Surgery, Fudan University Shanghai Cancer Center, Shanghai, China; Cancer Hospital of Shantou University Medical College, Shantou, China; Tianjin Medical University Cancer Institute & Hospital, Tianjin, China; Jiangsu Cancer Hospital, Nanjing, China.
You Can Also Watch ctDNA for Colorectal Cancer – Early and Metastatic Settings by Oncodaily
Written by Aharon Tsaturyan MD


