Anal squamous cell carcinoma (SCCA) is a rare, largely HPV-associated malignancy in which outcomes after progression on chemotherapy remain poor. PD-1/PD-L1 inhibitors have produced modest but real activity in previously treated disease, creating a rationale to test whether intensifying immune checkpoint blockade—specifically adding CTLA-4 inhibition—could increase response depth, durability, or survival. The biologic argument is familiar from other tumors: PD-1 blockade can reinvigorate exhausted T cells, whereas CTLA-4 blockade can broaden priming and expand T-cell repertoires. However, whether that translates into clinically meaningful benefit in refractory anal cancer had not been tested in a randomized design.
Study Design and Methods
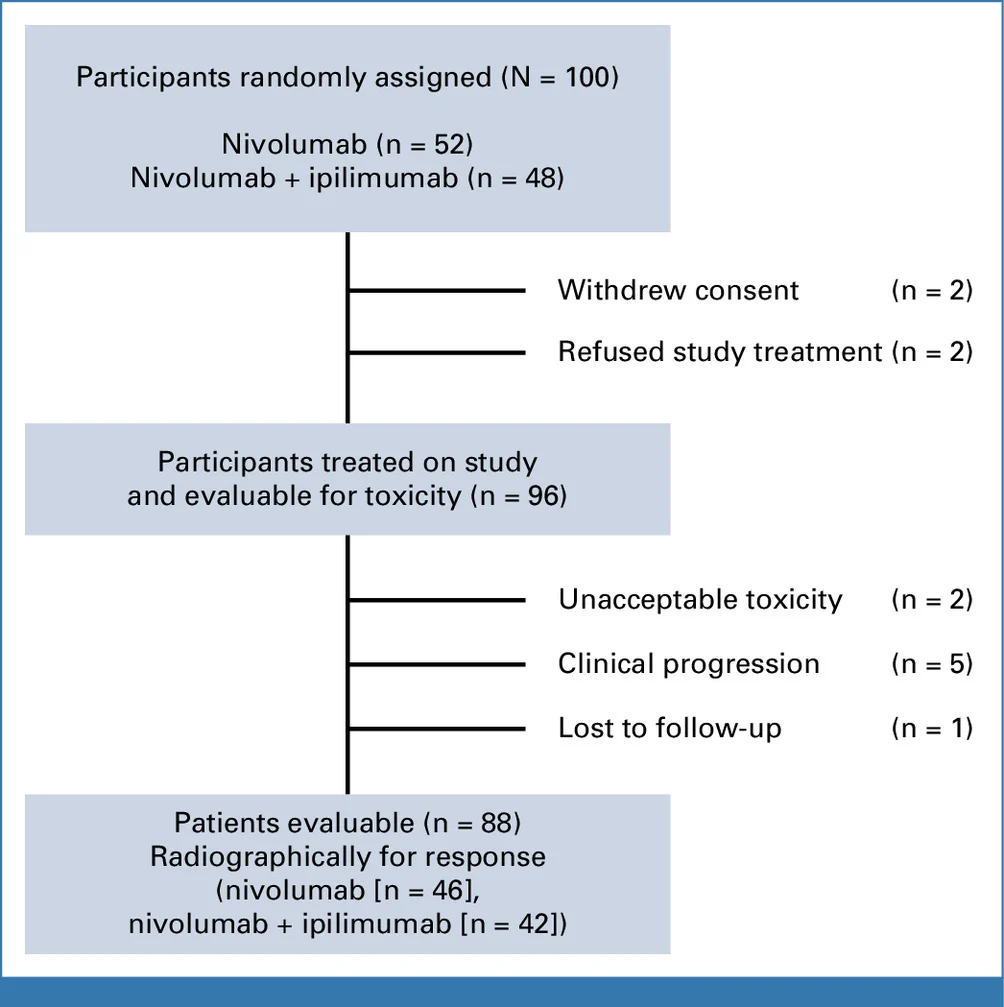
NCI9673 (Part B) was a multi-institutional, randomized phase II trial conducted within the NCI Experimental Therapeutics Clinical Trials Network (ETCTN). The study enrolled 100 patients with refractory, incurable squamous cell carcinoma of the anal canal, including those with metastatic disease or unresectable locally advanced tumors, all of whom had measurable disease by RECIST v1.1.
Eligible patients were required to have received prior definitive chemoradiation and at least one line of systemic therapy for incurable disease, or to have experienced early metastatic relapse within six months of completing chemoradiation. Prior exposure to immune checkpoint inhibitors was not permitted. Patients living with HIV were eligible provided infection was well controlled; however, no HIV-positive patients were ultimately enrolled. Importantly, PD-L1 expression and HPV status were not mandated for study entry, reflecting a biomarker-unselected population.
Participants were randomized in a 1:1 ratio to receive either:
- Nivolumab monotherapy at 480 mg intravenously every 4 weeks, or
- Combination therapy with nivolumab (480 mg IV every 4 weeks) plus low-dose ipilimumab (1 mg/kg IV every 8 weeks).
The primary endpoint was progression-free survival (PFS). Secondary endpoints included objective response rate (ORR), overall survival (OS), and the incidence of grade ≥3 treatment-related adverse events (TRAEs). In parallel, the trial incorporated correlative translational analyses, with longitudinal immune profiling of tumor tissue and peripheral blood using high-dimensional flow cytometry, focusing on baseline and on-treatment assessments at week 9.
From a statistical standpoint, the study was designed with a one-sided alpha of 0.1 and powered to detect a clinically meaningful improvement in PFS. This relatively permissive design is notable, as the addition of ipilimumab failed to meet the primary efficacy endpoint even under these assumptions, underscoring the robustness of the negative result.
Patient Cohort
- 100 treated participants (received ≥1 dose) enrolled between Oct 2018 and May 2024.
- Arms: 52 nivolumab alone, 48 nivolumab + ipilimumab.
- Demographics: majority female (77%) and Caucasian (96%); mean age ~60 years in both arms.
- Data cut-off: June 18, 2024.
- Median follow-up: 31.3 months, with 85 PFS events observed—so outcomes were observed with substantial follow-up time for a phase II study.
Results
Progression-Free Survival (Primary Endpoint)
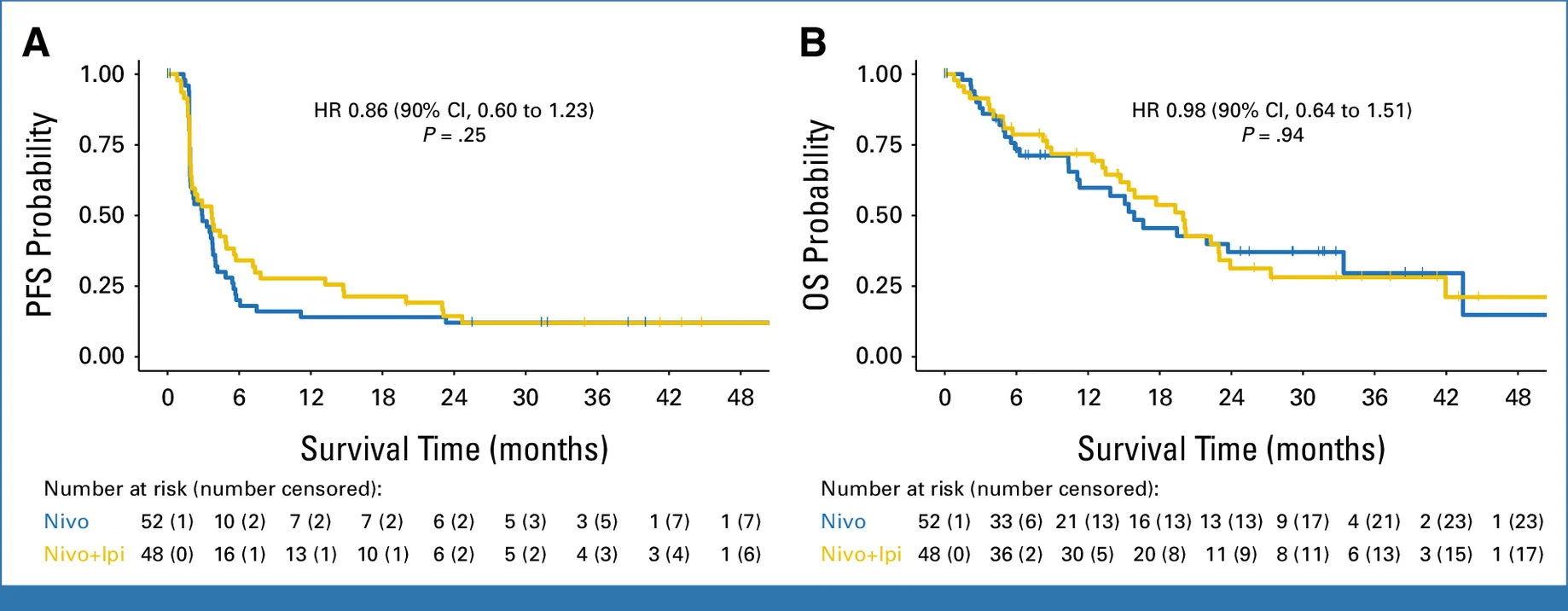
The addition of ipilimumab to nivolumab did not result in a statistically significant improvement in progression-free survival. Median PFS was 2.9 months (90% CI, 1.9–3.8) with nivolumab alone compared with 3.7 months (90% CI, 2.0–5.6) for the nivolumab–ipilimumab combination. The corresponding hazard ratio was 0.86 (95% CI, 0.60–1.23), with a one-sided log-rank P value of 0.25, failing to meet the predefined efficacy threshold.
Although a numerical prolongation of PFS was observed with dual checkpoint blockade, the magnitude of benefit was modest and not statistically robust. The broad overlap of confidence intervals and a hazard ratio crossing unity reinforce the conclusion that no reliable population-level advantage was achieved by adding CTLA-4 inhibition.
An exploratory view of landmark PFS rates provides additional nuance. At six months, approximately 20% of patients receiving nivolumab alone remained progression-free compared with 34% in the combination arm, albeit with wide 90% confidence intervals. This pattern suggests that a small subset of patients may derive more durable disease control with dual checkpoint blockade; however, this apparent tail effect was insufficient to translate into a meaningful or statistically significant benefit across the overall study population.
Objective Response
Response rates were similar between arms:
ORR
- Nivolumab: 17.4% (95% CI 9.1–31)
- Nivo/ipi: 21.5% (95% CI 12–36)
- P = 0.89 (no difference)
Complete responses occurred in both arms (3 with nivolumab, 2 with combo), and when CR happened, it tended to be durable—but the frequency of deep responders was low and not enriched meaningfully by ipilimumab.
Overall Survival
OS numerically favored the combination but was not meaningfully different statistically:
Median OS
- Nivolumab: 15.9 months (90% CI 11.3–33.4)
- Nivo/ipi: 20.0 months (90% CI 15.4–23)
HR: ~0.98 (90% CI 0.64–1.51)
Interpretation nuance: OS can be heavily influenced by subsequent therapies post-progression (which were not fully captured here). The absence of a clear PFS/ORR difference makes it hard to attribute any OS numerical difference to the experimental regimen.
Safety and Tolerability
Dual checkpoint blockade was associated with higher toxicity without a clear efficacy advantage. Grade ≥3 treatment-related adverse events occurred in 12% of patients receiving nivolumab alone versus 25% with nivolumab plus ipilimumab.
Severe immune-related events were more frequent in the combination arm and included pneumonitis, with one grade 5 fatal case following a complete response, underscoring the potential severity of immune toxicity. Immune-mediated hyperglycemia, including cases consistent with new-onset type 1 diabetes requiring insulin, was also observed with dual therapy.
Common all-grade adverse events were consistent with known checkpoint inhibitor profiles (fatigue, diarrhea, rash, endocrine dysfunction). Clinical implication: In refractory metastatic anal cancer, adding ipilimumab increased immune toxicity without delivering meaningful clinical benefit, resulting in an unfavorable risk–benefit profile for routine use.
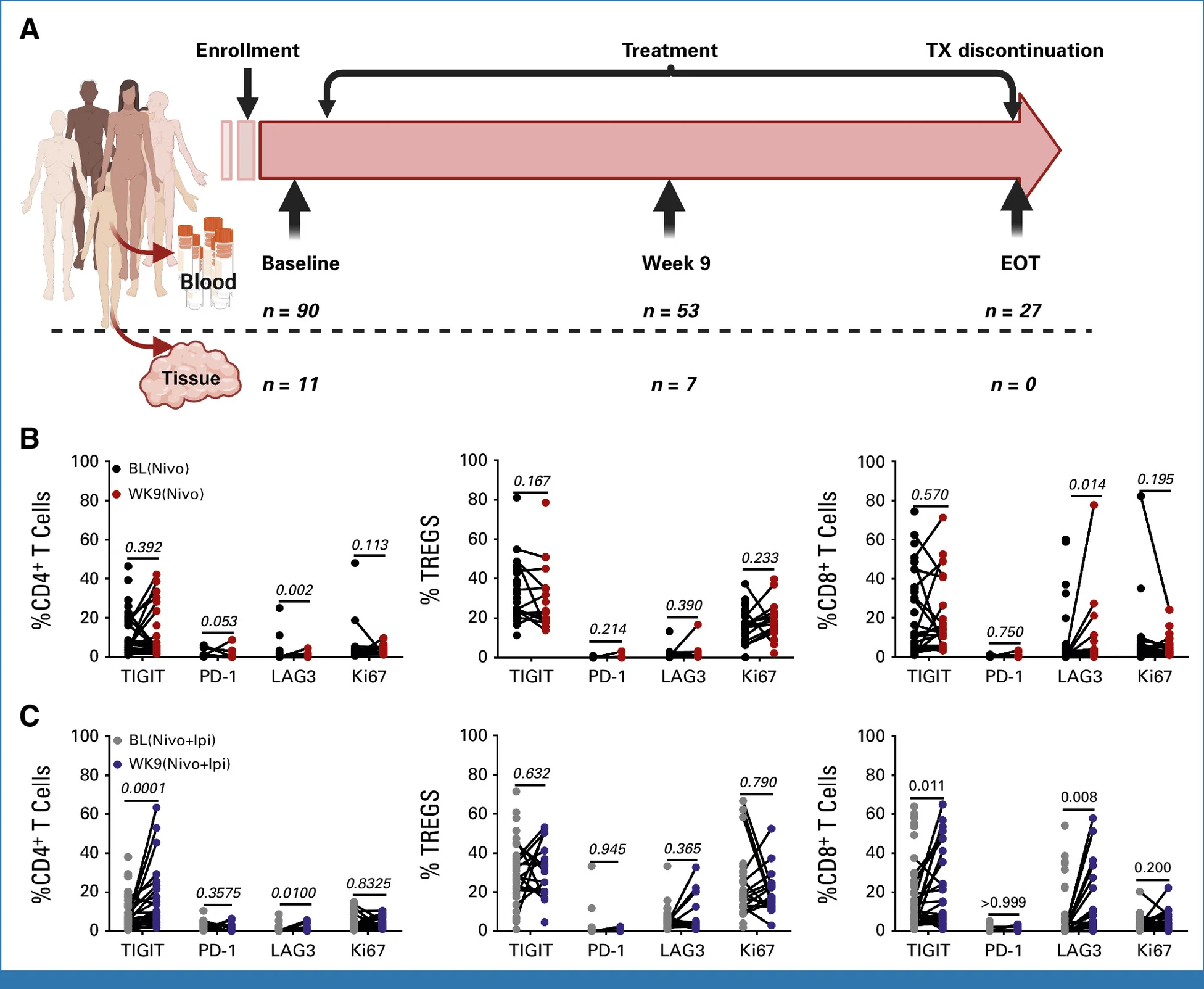
Translational Insights
Immune profiling helps explain why dual checkpoint blockade failed to improve outcomes. Tumor tissue analyses were limited by scarce paired biopsies and did not show consistent changes in intratumoral activation or exhaustion markers.
In contrast, peripheral blood profiling revealed a clear signal. By week 9, patients receiving nivolumab plus ipilimumab showed a selective increase in TIGIT⁺ CD8⁺ T cells, a pattern less evident with nivolumab alone. While other inhibitory receptors (e.g., LAG-3) changed across both arms, TIGIT upregulation was the distinguishing feature of dual blockade.
Interpretation: TIGIT is an inhibitory receptor linked to T-cell dysfunction and immune escape. Its induction with CTLA-4 plus PD-1 blockade suggests a compensatory resistance mechanism, potentially offsetting any incremental immune activation while increasing toxicity. This provides a biologic rationale for the lack of clinical benefit and positions TIGIT as a future therapeutic target—a hypothesis-generating insight rather than a practice-changing conclusion.
Practical interpretation
In refractory metastatic anal SCC, nivolumab remains a reasonable immunotherapy option with modest response rates and a tail of durable benefit. However, in a randomized setting, adding low-dose ipilimumab did not meaningfully improve response, PFS, or OS, and it increased clinically relevant immune-related toxicity. Translational immune profiling suggests that **dual PD-1/CTLA-4 blockade may trigger compensatory inhibitory pathways—particularly TIGIT upregulation on circulating CD8+ T cells—**providing a plausible mechanistic explanation for the lack of incremental efficacy and offering a rational direction for next-generation combinations.
You Can Read All Article Here