Ampullary adenocarcinoma (AAC) is a rare gastrointestinal malignancy, accounting for approximately 0.2% of digestive malignancies. Owing to its anatomical location at the ampulla of Vater, AAC is more frequently amenable to curative-intent surgical resection than pancreatic ductal adenocarcinoma. Nevertheless, long-term outcomes remain unsatisfactory, with reported 5-year overall survival rates ranging from 30% to 67%, largely due to high postoperative recurrence rates.
To date, the role of adjuvant chemotherapy in AAC remains controversial. While single-agent fluoropyrimidines or gemcitabine are commonly used, practices are heterogeneous and supported by limited prospective evidence. Observational and retrospective data suggest that adjuvant therapy may benefit patients with intermediate- and high-risk disease, particularly those with advanced tumor stage, pancreatobiliary, mixed, or undetermined histological subtypes. However, no phase III trial has been specifically dedicated to defining the optimal adjuvant strategy in resected AAC.
The ongoing AMPIRINOX (PRODIGE 98) trial was designed to address this unmet need by evaluating whether adjuvant modified FOLFIRINOX (mFOLFIRINOX) can improve disease-free survival compared with single-agent chemotherapy with capecitabine or gemcitabine in patients with resected ampullary adenocarcinoma.

You can also read about Chemotherapy Success Rate: What It Means and When It Works Best on OncoDaily.
Study Design and Methods
AMPIRINOX (PRODIGE 98; FFCD 2105) is a multicenter, open-label, randomized phase III trial conducted within the PRODIGE intergroup and sponsored by the Fédération Francophone de Cancérologie Digestive (FFCD), with funding from the French National Cancer Institute (INCa). The trial is registered under ClinicalTrials.gov identifier NCT06813976 and is currently recruiting. Recruitment started in April 2025.
Eligible patients are adults aged 18 to 79 years who have undergone macroscopically complete (R0 or R1) surgical resection of histologically confirmed ampullary adenocarcinoma. Patients must have no evidence of metastatic disease on cross-sectional imaging (CT, or CT plus MRI if CT is contraindicated) performed within 4 weeks prior to inclusion. Key inclusion criteria include WHO performance status 0–1 (restricted to 0 in patients older than 75 years) and CA19-9 at inclusion < 180 UI/mL. Patients with prior neoadjuvant chemotherapy, pT1N0M0 tumors, significant peripheral neuropathy, poor nutritional status, or pharmacogenetic contraindications to fluoropyrimidines or irinotecan, including dihydropyrimidine dehydrogenase deficiency and homozygosity for UGT1A1*28, are excluded.
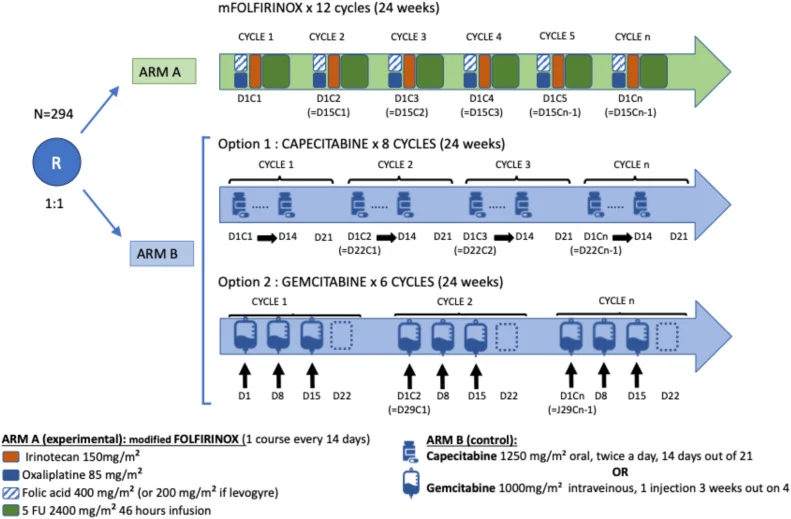
Participants are randomized in a 1:1 ratio within 12 weeks after surgery and at least 1 week before the first chemotherapy dose to receive one of two adjuvant strategies for a total duration of 24 weeks:
- Experimental arm: Intravenous mFOLFIRINOX administered every two weeks for 12 cycles (oxaliplatin, irinotecan, leucovorin, and continuous-infusion 5-fluorouracil), with systematic G-CSF strongly recommended.
- Control arm: Single-agent chemotherapy with capecitabine or gemcitabine, according to investigator choice.
Randomization is stratified by center, histological subtype (intestinal vs pancreatobiliary, mixed, or undetermined), TNM stage (I–II vs III), differentiation grade, and CA19-9 level at inclusion (<90 vs ≥90 UI/mL).
Endpoints and Objectives
The primary objective of AMPIRINOX is to determine whether adjuvant mFOLFIRINOX improves disease-free survival (DFS) compared with single-agent chemotherapy after surgical resection of ampullary adenocarcinoma.
DFS is defined as the time from randomization to first documented relapse (local or metastatic) or death from any cause, with relapse assessed by the investigator according to RECIST v1.1 criteria; second primary cancers are not considered DFS events.
Secondary endpoints include overall survival, treatment completion rates, safety and toxicity profiles, quality of life assessed by EORTC QLQ-C30 and PAN26 questionnaires, and prespecified subgroup analyses by histological subtype, stage, and prognostic score.
Statistical Considerations
The trial is powered to detect an absolute improvement in 2-year DFS from 60% in the control arm to 71% in the experimental arm, corresponding to a hazard ratio of 0.67. Assuming a two-sided α of 5%, 80% power, and a total follow-up of 48 months, 203 DFS events are required. Accounting for potential attrition, the total planned enrollment is 294 patients (147 per arm).
Two interim analyses are scheduled at 33% and 60% of events using O’Brien–Fleming boundaries with both alpha- and beta-spending for efficacy and futility. All analyses will be conducted on an intention-to-treat basis, with alternative methods such as restricted mean survival time considered if proportional hazards assumptions are not met.
AMPIRINOX vs Prior Trials
Only two phase III trials have previously included AAC patients in the adjuvant setting. The ESPAC-3 periampullary trial did not demonstrate a clear survival advantage for adjuvant chemotherapy over observation in the overall population, although multivariable analysis favored single-agent chemotherapy. The ASCOT trial in biliary tract cancers showed a benefit of S-1 over observation, with a signal in the AAC subgroup, but was not specifically designed for this disease.
Retrospective cohort studies suggest that combination chemotherapy may provide superior survival outcomes compared with single-agent regimens in resected AAC. Meanwhile, mFOLFIRINOX has demonstrated substantial survival benefits in both metastatic and adjuvant pancreatic cancer, supporting its evaluation in AAC despite biological heterogeneity across histological subtypes.
AMPIRINOX is the first randomized phase III trial specifically designed to compare combination versus single-agent adjuvant chemotherapy exclusively in ampullary adenocarcinoma.
Translational and Ancillary Studies
A major strength of AMPIRINOX is its extensive translational research program. Every patient will have access to comprehensive molecular profiling, including whole-genome and RNA sequencing through the French national genomic medicine initiative. FFPE tumor tissue will be collected, and optional ancillary studies will evaluate circulating tumor DNA and extracellular vesicles, alongside serial CA19-9 measurements, to explore prognostic and predictive biomarkers of recurrence and treatment response.
Additional analyses will investigate molecular subgroups, transcriptomic signatures of chemosensitivity, and interobserver variability in histopathological classification, with the goal of refining risk stratification and future therapeutic decision-making.
Conclusion
The AMPIRINOX (PRODIGE 98) trial addresses a critical evidence gap in the management of resected ampullary adenocarcinoma. By directly comparing adjuvant mFOLFIRINOX with single-agent chemotherapy in a dedicated phase III setting, this study aims to define an evidence-based postoperative strategy for a rare but aggressive disease. Through integrated molecular analyses and long-term follow-up, AMPIRINOX is expected to substantially advance both clinical practice and biological understanding of ampullary adenocarcinoma.
The study design and protocol report are published in Digestive and Liver Disease (Articles in Press, January 31, 2026).