BRAF V600E–mutated metastatic colorectal cancer (mCRC) represents a biologically aggressive subtype accounting for approximately 8–10% of all mCRC cases and is associated with poor prognosis and limited sensitivity to conventional chemotherapy. The therapeutic landscape changed significantly with the BEACON CRC trial, which established encorafenib plus cetuximab (ENCO-CET) as the standard of care in previously treated patients, demonstrating improved response rates, progression-free survival (PFS), and overall survival (OS) compared with chemotherapy-based regimens.
However, cetuximab can cause infusion-related reactions (IRRs); while severe events are uncommon, grade ≥3 IRRs occur in ~2–5% of treated patients despite premedication and may be life-threatening, requiring treatment discontinuation. This creates a major clinical challenge, as BRAF inhibition in colorectal cancer requires concomitant EGFR blockade to achieve meaningful antitumor activity.
In this context, results from an international multicenter analysis titled “Switch from cetuximab to panitumumab during encorafenib-based therapy in BRAF V600E–mutated metastatic colorectal cancer: an international multicenter analysis from the AGEO group” were available online in Clinics and Research in Hepatology and Gastroenterology on Dec 16, 2025; published in Volume 50, Issue 1 (January 2026).
Authors: Annalice Gandini, Victoria Probst, Matteo Landi, Maria Caterina De Grandis, Chiara Cremolini, Sara Lonardi, Paul Girot, Marie Decraecker, Alessandro Passardi, Lisa Salvatore, Alessandro Pastorino, Jeremy C Jones, Lucien Grados, Lina Sayah, Isabelle Trouilloud, David Tougeron, Julien Taieb.
Methods and Endpoints
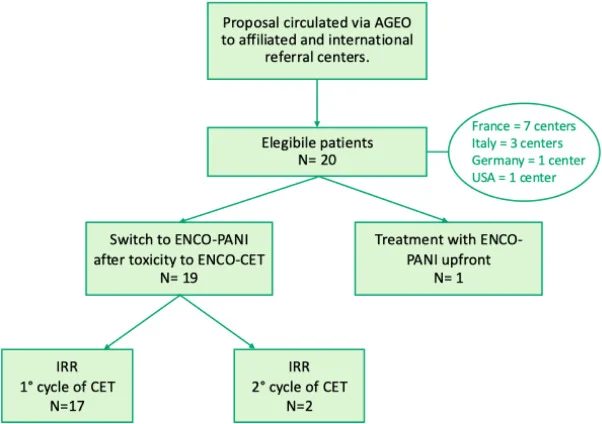
This retrospective study included patients with BRAF V600E–mutated mCRC identified through an AGEO registry and collaborating international centers. Patients had initiated encorafenib-based therapy with cetuximab and subsequently switched to panitumumab following an infusion-related reaction, or received ENCO-PANI upfront by choice.
Tumor responses were assessed locally according to RECIST 1.1 criteria. The primary efficacy endpoints were objective response rate (ORR) and disease control rate (DCR). Secondary endpoints included progression-free survival, overall survival, and safety. Survival outcomes were estimated using Kaplan–Meier methodology, and toxicities were graded according to CTCAE version 5.0.
Results
Twenty patients were included across 12 centers in four countries. The median age was 66 years, most patients had right-sided primary tumors, and one quarter had dMMR/MSI disease. The majority were treated in the second-line setting.
Nineteen patients started ENCO–CET and switched to ENCO–PANI at cycle 2 or 3 after a cetuximab-related IRR (predominantly grade 3–4), most often during the first infusion. One patient received ENCO-PANI upfront. Encorafenib was administered at the standard 300 mg daily dose in nearly all patients, while panitumumab was given bi-weekly at 6 mg/kg without premedication.
Efficacy Outcomes
Treatment with ENCO-PANI resulted in clinically relevant antitumor activity:
- Objective response rate: 25%
- Disease control rate: 85%
- Best responses: partial response in 5 patients, stable disease in 12 patients, progressive disease in 3 patients
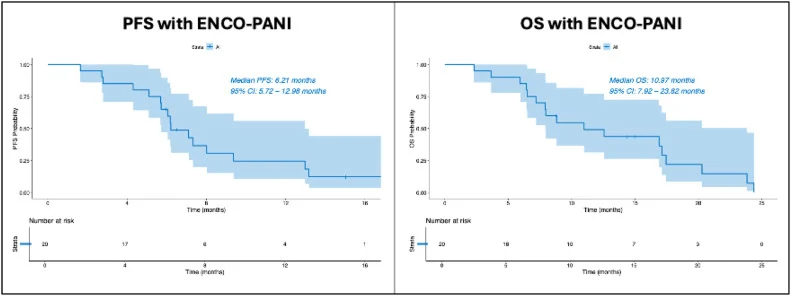
With a median follow-up of 15 months, median PFS was 6.2 months, and median OS 10.97 months (~11 months). These outcomes are broadly comparable to historical results reported with ENCO-CET in the BEACON trial, acknowledging the limitations of retrospective and cross-trial comparisons.
Safety
ENCO-PANI demonstrated a manageable safety profile. Any-grade adverse events occurred in 75% of patients and were mostly grade 1–2. The most frequent AEs were skin rash (60%) and diarrhea (25%), followed by asthenia and hypomagnesemia (10% each); arthralgia, nausea, and liver enzyme elevation were also reported.
Notably:
- No infusion-related reactions were observed with panitumumab
- No treatment-related deaths occurred
- Only one patient discontinued ENCO-PANI due to toxicity
Conclusion
In this largest reported real-world series to date, encorafenib plus panitumumab demonstrated meaningful efficacy and a favorable safety profile in patients with BRAF V600E–mutated metastatic colorectal cancer who were unable to continue cetuximab due to infusion-related reactions. With an 85% disease control rate, median PFS of 6.2 months, and median OS of approximately 11 months, ENCO-PANI appears to preserve the clinical benefit of dual BRAF and EGFR inhibition while eliminating the risk of recurrent IRRs.
These findings support ENCO-PANI as a valid alternative therapeutic option in this setting and reinforce the importance of maintaining EGFR blockade in BRAF-mutated colorectal cancer. Prospective studies and additional real-world data will be important to further define its role, including potential use in earlier lines of therapy.
Read full article here.
You can also read about BRAF-Mutant Colorectal Cancer Active and Recruiting Trials You Need to Know in 2025 on OncoDaily.