Microsatellite instability–high (MSI-H) colon cancer represents a biologically distinct subtype characterized by defective DNA mismatch repair, high tumor mutational burden, and immune-rich microenvironments that confer robust responsiveness to immune checkpoint inhibition.
Peritoneal metastases, however—particularly isolated peritoneal metastases (iPM)—pose unique therapeutic challenges. They are associated with limited vascularization, impaired drug penetration, and poor outcomes under conventional chemotherapy. Historically, cytoreductive surgery (CRS) and heated intraperitoneal chemotherapy (HIPEC) have been reserved for selected patients, but outcomes remain unsatisfactory.
The optimal management of MSI-H colon cancer with iPM remains undefined, and the role of surgery in the era of immunotherapy is unclear. To address this gap, Siolas et al. leveraged the U.S. National Cancer Database (NCDB) to examine outcomes associated with immunotherapy, chemotherapy, and surgical resection in this rare and clinically challenging population.
Study Design and Methods
This retrospective cohort study included 598 patients diagnosed between 2016–2021 with MSI-H colon cancer and iPM, identified from 456,000 colon cancer cases in the NCDB.
Eligible patients received systemic therapy and were categorized into three groups:
- Immunotherapy alone (IO)
- Chemotherapy alone (CT)
- Combination chemotherapy + immunotherapy (CT + IO)
Treatment patterns, surgical interventions, and survival outcomes were analyzed.
The primary endpoint was overall survival (OS), assessed using Kaplan–Meier and Cox proportional hazardsmodels adjusted for age, comorbidities, and histology.
Immunotherapy utilization trends (2016–2022) were also evaluated to assess adoption following integration of checkpoint blockade into NCCN guidelines.
Results
Among the 598 patients:
- 22% received immunotherapy alone,
- 43% chemotherapy alone, and
- 35% combination CT + IO.
Most patients (76%) underwent surgical resection, and 93% of systemic therapy use occurred postoperatively (adjuvant setting).
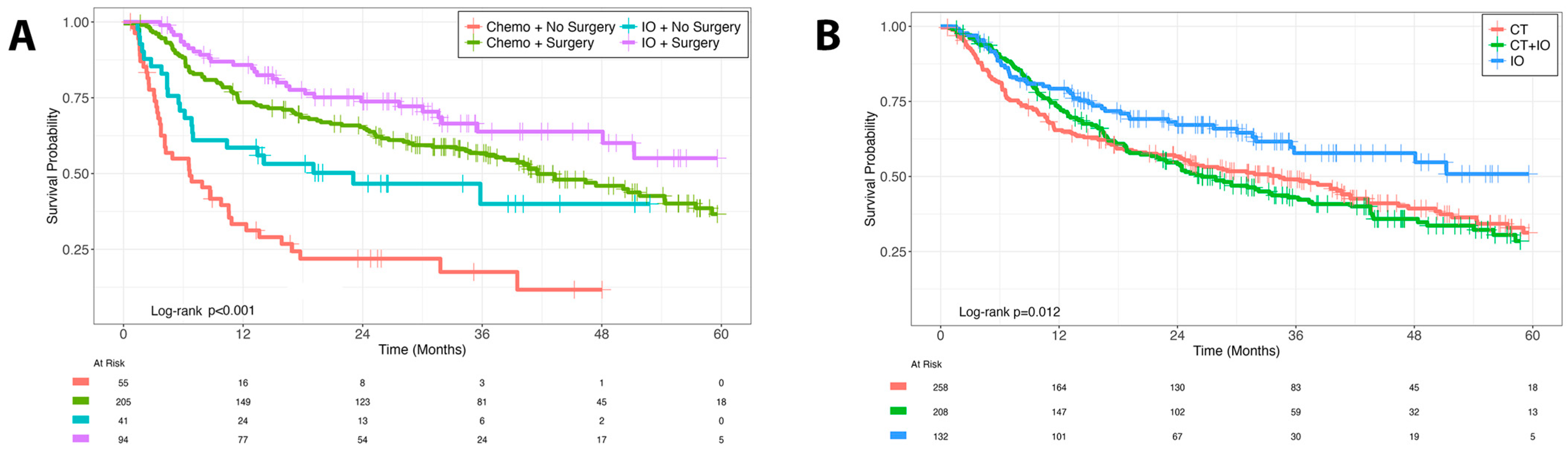
Immunotherapy recipients were older (median 75 years vs. 67 years; p < 0.001) and had higher comorbidity scores, yet demonstrated superior outcomes.
Key findings included:
- Median OS: 33 months with IO vs. 18 months with CT (p < 0.001)
- Multivariable HR for IO vs. CT: 0.46 (p < 0.001)
- Surgical resection (with or without metastatectomy): HR 0.40–0.41 (p < 0.001)
- Combined surgery + immunotherapy: Best 5-year OS 55.1% (p < 0.001)
- CT + IO combination: No additional benefit over chemotherapy alone (HR 0.97; p = 0.80)
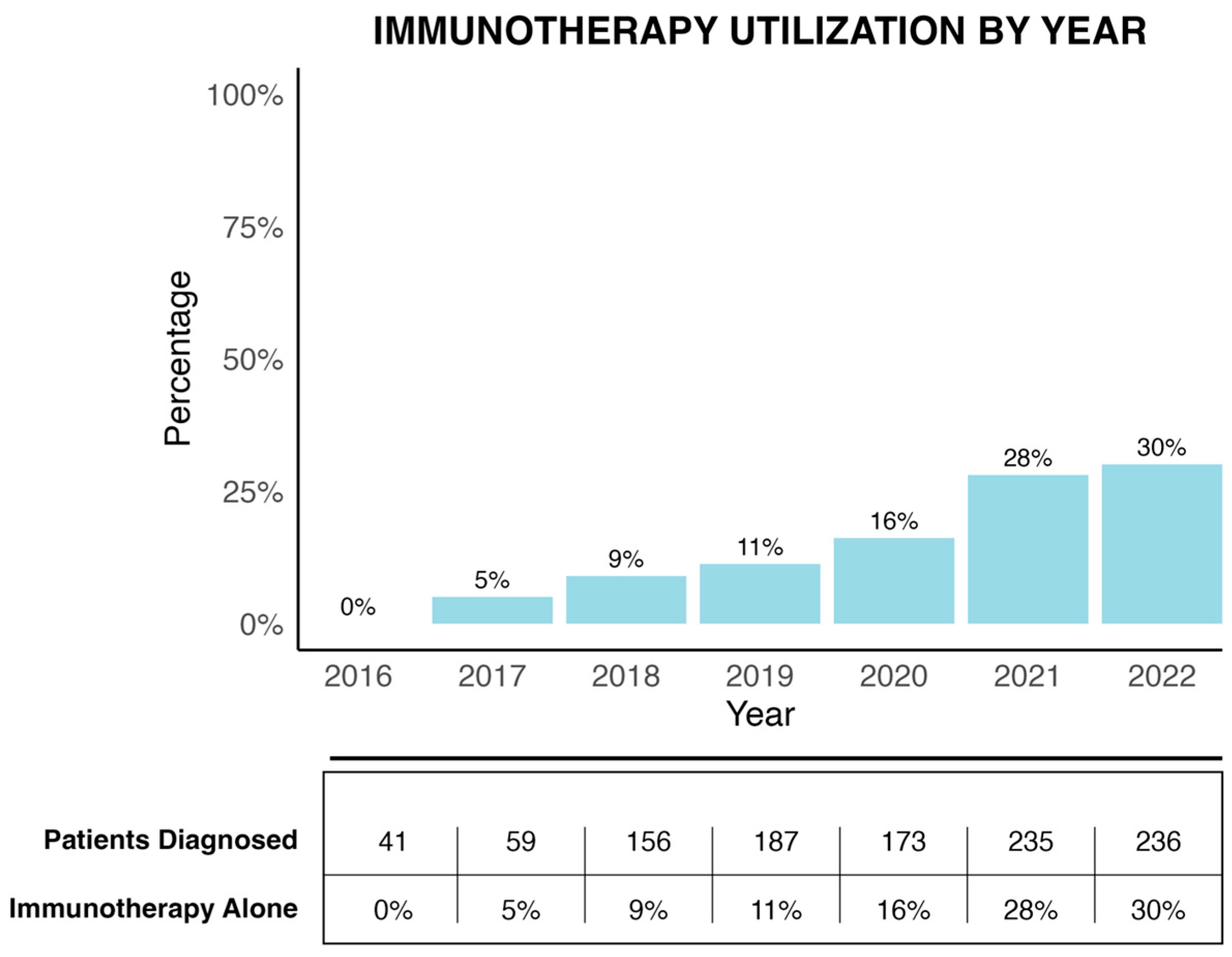
Use of immunotherapy increased from 0% in 2016 to 30% by 2022, reflecting evolving clinical adoption after KEYNOTE-177, but significant underutilization persisted—especially outside academic centers.
Discussion
This analysis provides the first population-level evidence that immune checkpoint blockade significantly improves survival in patients with MSI-H colon cancer and isolated peritoneal metastases, a subgroup historically considered chemo-resistant and often excluded from immunotherapy trials.
The study further demonstrates that surgery remains a powerful prognostic factor, even in metastatic disease. When combined with immunotherapy, surgical resection achieved the greatest long-term survival, suggesting biological synergy between immune activation and cytoreductive control.
Mechanistically, immunotherapy may overcome the limited drug penetration typical of peritoneal metastases by generating systemic cytotoxic T-cell activation effective in compartmentalized tumor sites. Additionally, checkpoint blockade before or after resection may facilitate immune memory formation and eradication of residual micrometastatic disease.
Importantly, despite older age and higher comorbidity, patients treated with immunotherapy experienced superior outcomes, reinforcing its efficacy and tolerability in real-world practice.
These results align with emerging data from ATOMIC (adjuvant atezolizumab + chemotherapy) and early-phase studies exploring perioperative immunotherapy in MSI-H colorectal cancer, collectively supporting integration of checkpoint inhibitors across the disease continuum.
Limitations
As a retrospective NCDB analysis, treatment allocation was non-randomized, and detailed molecular data (e.g., RAS, BRAF, HER2 status) were unavailable. The NCDB’s “immunotherapy” variable includes biologic agents, though analytic stratification minimized misclassification. Data on progression-free survival and Peritoneal Cancer Index (PCI) were not captured, limiting granularity of disease-burden assessment. Nonetheless, the large sample and robust multivariable modeling strengthen the validity of the observed associations.
Conclusion
Immunotherapy substantially improves survival compared with chemotherapy in MSI-H colon cancer with isolated peritoneal metastases.
Surgical resection—especially when combined with checkpoint blockade—yields the most favorable outcomes, supporting a multimodal treatment paradigm rather than systemic therapy alone.
These findings challenge conventional reluctance toward surgery in metastatic disease and highlight the need for prospective trials to define optimal sequencing, timing, and patient selection for combined immunotherapy-surgical strategies in MSI-H peritoneal-limited colorectal cancer.
Key Takeaway Messages
- Immunotherapy doubles median OS vs. chemotherapy (33 vs. 18 months; HR 0.46).
- Surgery + immunotherapy confers the highest survival (5-year OS 55%).
- Chemotherapy + immunotherapy offers no incremental benefit.
- Utilization of IO in this setting rose to 30% by 2022 but remains suboptimal.
- Findings support integrated multimodal management for MSI-H colon cancer with peritoneal metastases, warranting prospective validation.
You Can Read Full Article Here