
ESMO GI 2025 Highlights: OrigAMI-1 Trial on Amivantamab Rechallenge in mCRC
The OrigAMI-1 trial (NCT05379595) was presented by Dr. Samuel J. Klempner during the ESMO GI 2025 conference, where he shared significant findings on rechallenge therapy for metastatic colorectal cancer (mCRC). The trial focused on the use of amivantamab, an EGFR-MET bispecific antibody, in patients with left-sided, RAS/BRAF wild-type mCRC who had previously progressed on EGFR inhibitors (EGFRi). The study evaluated the efficacy of amivantamab monotherapy, particularly examining how the time interval between prior EGFRi treatment and the initiation of amivantamab rechallenge impacted patient outcomes. Dr. Klempner highlighted that a longer gap between EGFRi withdrawal and rechallenge was associated with improved response rates, progression-free survival, and overall survival.
Background
In metastatic colorectal cancer (mCRC), resistance to EGFR inhibitors (EGFRi) is common, with resistant clones decaying at a mutational half-life of approximately 4.4 months (Parseghian Ann Onc 2019). The OrigAMI-1 trial (NCT05379595) assessed the effectiveness of amivantamab, a bispecific antibody targeting both EGFR and MET, as a rechallenge therapy for left-sided, RAS/BRAF wild-type mCRC patients who previously progressed on EGFRi.
Methods
Cohort B of the OrigAMI-1 study evaluated amivantamab monotherapy in patients who had progressed after 2-3 prior lines of EGFRi treatment. Participants were assessed for KRAS, NRAS, BRAF, and EGFR ectodomain mutations by central ctDNA testing. The primary endpoint was objective response rate (ORR), assessed by investigators per RECIST v1.1. Patients were grouped based on the time from the last EGFRi dose: those rechallenged within 8.8 months (shorter interval group) and those rechallenged after 8.8 months (longer interval group).
Results
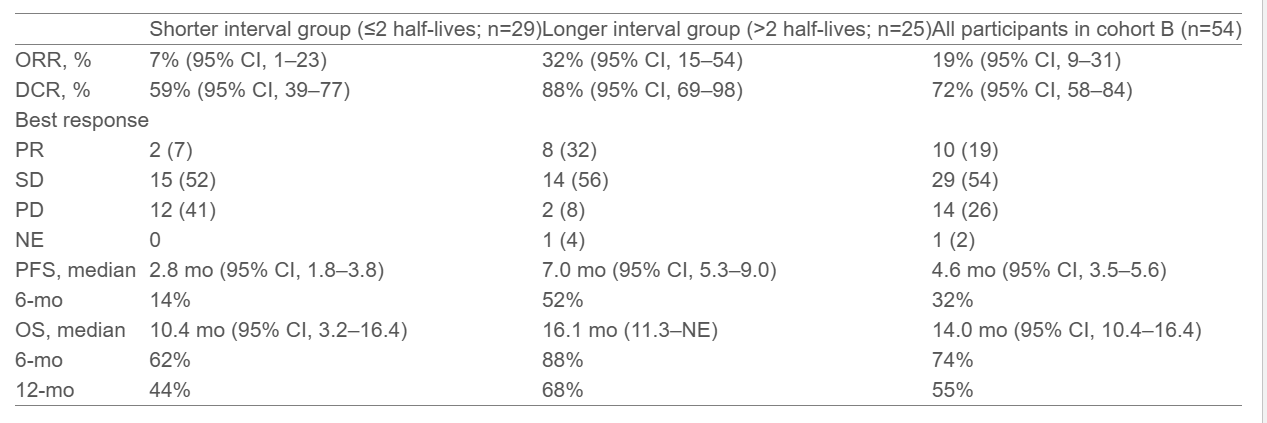
The results from the OrigAMI-1 trial (NCT05379595) presented at ESMO GI 2025 provide valuable insights into the efficacy of amivantamab rechallenge in patients with left-sided, RAS/BRAF wild-type metastatic colorectal cancer (mCRC) after prior EGFR inhibitor therapy. As of October 31, 2024, 54 patients were enrolled in the study, with a median follow-up of 13.7 months. The patients were divided into two groups based on the time elapsed between their prior EGFRi treatment and the initiation of amivantamab monotherapy: the shorter interval group (≤8.8 months) and the longer interval group (>8.8 months). Below are the key findings from the trial:
Objective Response Rate (ORR):
- Longer interval group: 32% (95% CI, 15–54)
- Shorter interval group: 7% (95% CI, 1–23)
Median Progression-Free Survival (PFS):
- Longer interval group: 7.0 months
- Shorter interval group: 2.8 months (nominal P=0.008)
Median Overall Survival (OS):
- Longer interval group: 16.1 months
- Shorter interval group: 10.4 months (nominal P=0.088)
Key Findings
- Objective Response Rate: 32% in the longer interval group vs. 7% in the shorter interval group.
- Progression-Free Survival (PFS): Median of 7.0 months in the longer interval group vs. 2.8 months in the shorter interval group.
- Overall Survival (OS): 16.1 months for the longer interval group vs. 10.4 months for the shorter interval group.
- Subgroup Analysis: The study indicated that patients who had a response to prior EGFRi were more likely to respond to amivantamab.
Key Takeaway Messages
- Rechallenge with amivantamab is associated with improved PFS and OS in patients who had at least a 9-month interval after prior EGFRi treatment.
- Shorter intervals between EGFRi and amivantamab rechallenge resulted in lower response rates and shorter survival outcomes.
Conclusion
Amivantamab demonstrated promising efficacy in rechallenging patients with left-sided, RAS/BRAF wild-type mCRC who previously progressed on EGFRi therapy. Longer intervals between treatments were associated with better outcomes, suggesting that rechallenge therapy may be more effective in patients with a longer period of EGFRi therapy withdrawal. Further studies are needed to explore these findings in a broader patient population.
You can read the full article here
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
