SERENA-6 Trial at ASCO 2025: Camizestrant Plus CDK4/6i Improves Outcomes in HR+/HER2– Advanced Breast Cancer with ctDNA-Detected ESR1 Mutations
At ASCO 2025, results presented by Turner Nicholas from the global Phase 3 SERENA-6 trial unveiled a landmark advancement in precision oncology: real-time ctDNA-guided treatment with camizestrant significantly improved outcomes in patients with HR+/HER2– advanced breast cancer (ABC) by targeting emerging ESR1 mutations before clinical progression.
Background
Hormone receptor-positive, HER2-negative advanced breast cancer is commonly treated with aromatase inhibitors (AIs) in combination with CDK4/6 inhibitors. However, resistance inevitably develops, often driven by acquired mutations in the ESR1 gene. These mutations activate the estrogen receptor independently of estrogen and render AIs ineffective. Detecting ESR1 mutations before radiographic progression could offer a critical therapeutic window to adapt treatment and delay resistance. Camizestrant, a next-generation selective estrogen receptor degrader (SERD), has shown anti-tumor efficacy in both ESR1-mutant and wild-type tumors. The SERENA-6 trial is the first global registrational trial to test whether ctDNA surveillance can guide an early therapeutic switch to camizestrant, thus improving patient outcomes before progression is visible on imaging.
Design and Methods
SERENA-6 enrolled patients with HR+/HER2– ABC who had received at least six months of first-line AI therapy (anastrozole or letrozole) combined with a CDK4/6 inhibitor (abemaciclib, palbociclib, or ribociclib). These patients underwent ctDNA testing every 2–3 months to monitor for the emergence of ESR1 mutations, coinciding with routine imaging. If an ESR1 mutation was detected and the patient had no radiologic disease progression, they were randomized 1:1 to either switch from AI to camizestrant (75 mg daily) while continuing their current CDK4/6 inhibitor, or to continue on their existing AI + CDK4/6i regimen. Both arms included a placebo to maintain blinding. 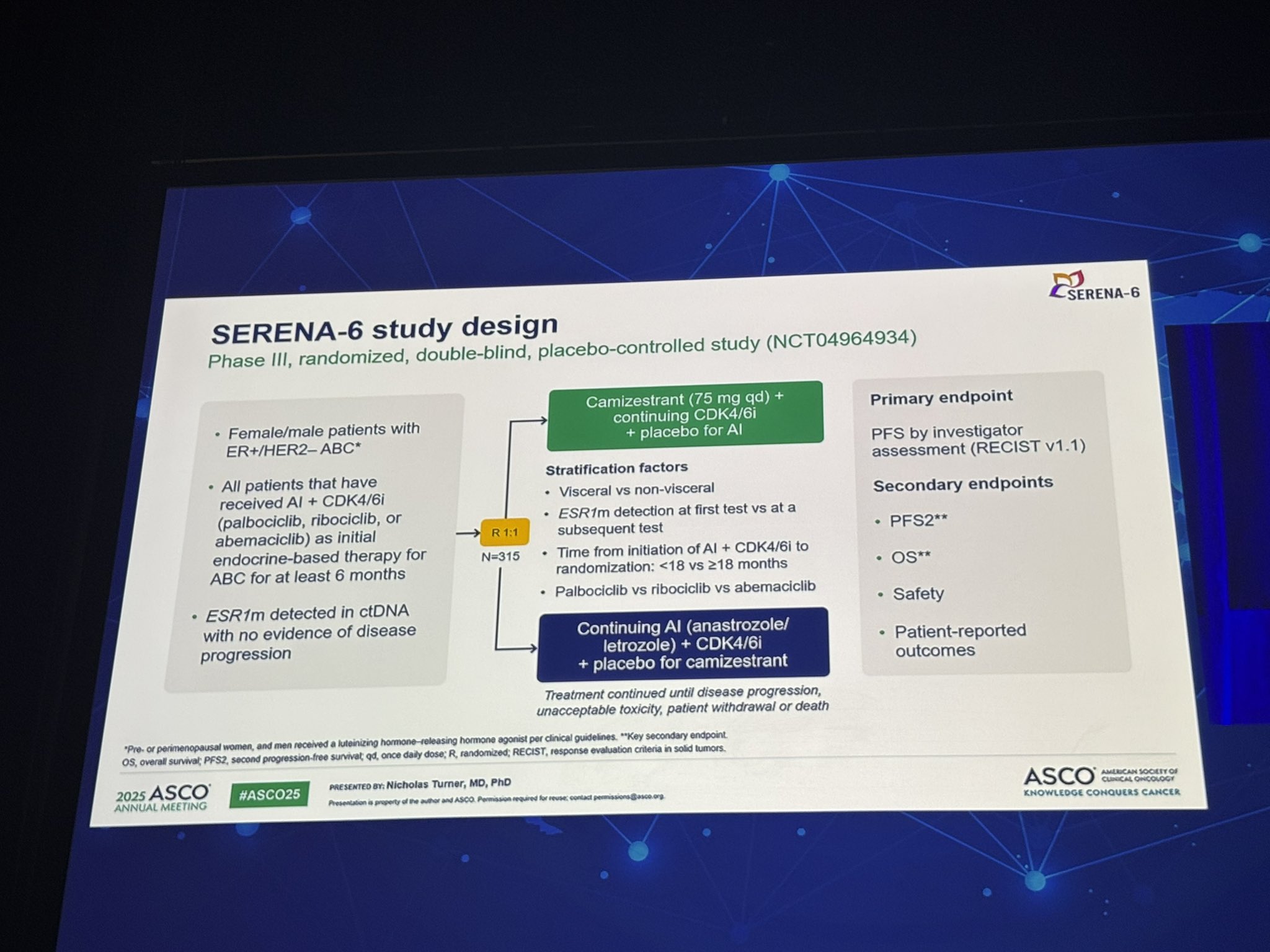
The primary endpoint was investigator-assessed progression-free survival (PFS) according to RECIST v1.1. Secondary endpoints included PFS2, overall survival (OS), and safety. The prespecified interim analysis included data up to the 28 November 2024 cutoff date.
Results of SERENA-6 Trial
Among 3,256 patients under ctDNA surveillance, 315 patients with newly detected ESR1 mutations and no progression were randomized—157 to the camizestrant arm and 158 to continue AI. Approximately half of the randomized patients had ESR1 mutations detected at their first ctDNA test. Baseline characteristics were well balanced across groups, and all patients remained on their original CDK4/6 inhibitor.
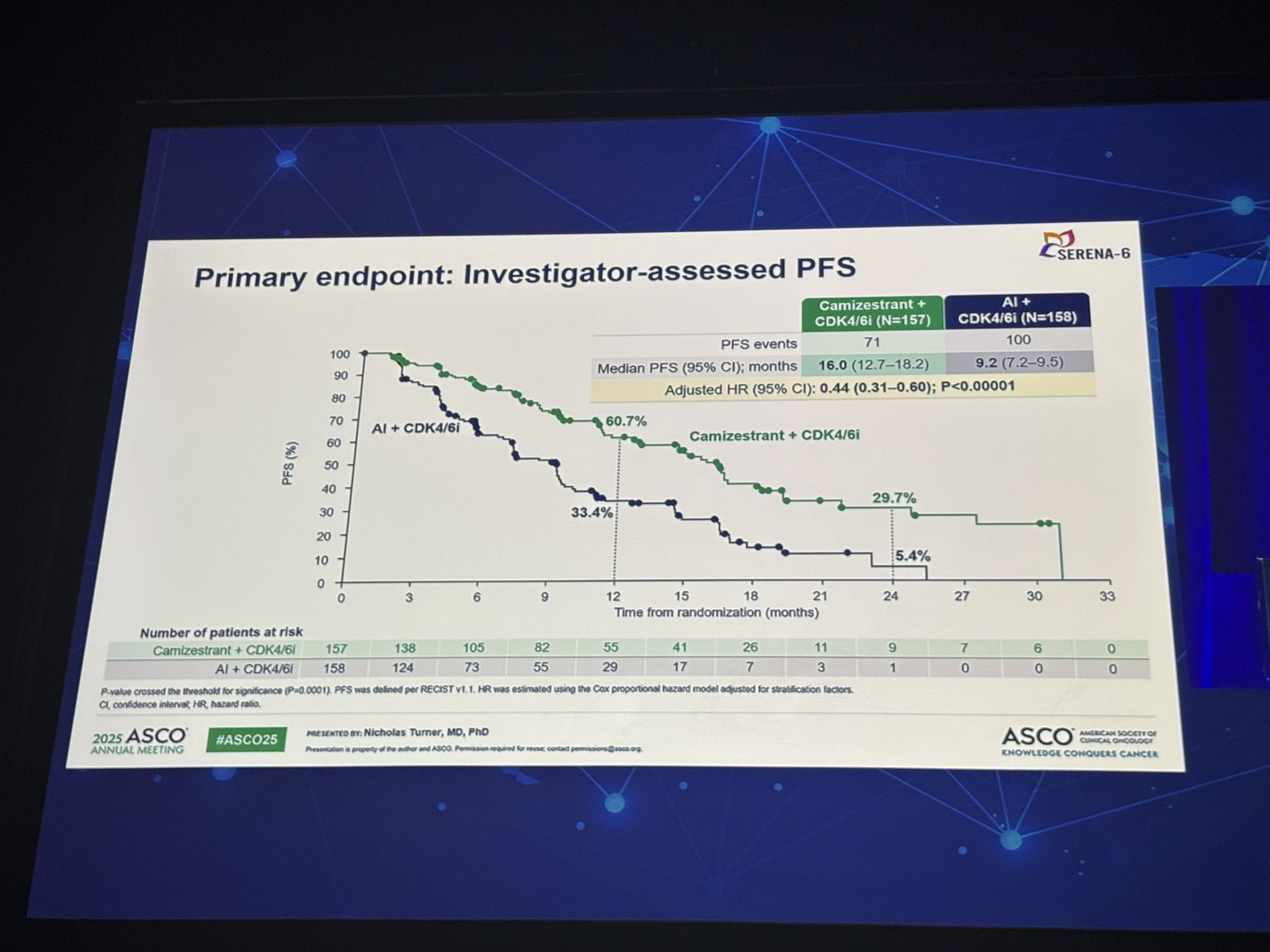
Primary Endpoint – Progression-Free Survival (PFS)
At the data cutoff (November 28, 2024), with 171 PFS events recorded, camizestrant demonstrated a significant improvement in PFS compared to continued AI
-
Median PFS was 16.0 months for camizestrant versus 9.2 months for AI.
-
Hazard ratio (HR) for progression or death was 0.44 (95% CI: 0.31–0.60; p<0.00001), indicating a 56% reduction in risk.
-
The benefit was consistent across all subgroups analyzed, including different CDK4/6 inhibitors.
PFS Rates Over Time
-
At 12 months, the PFS rate was 60.7% (95% CI 51.1–69.0) with camizestrant vs 33.4% (95% CI 24.9–42.2) with AI.
-
At 24 months, the PFS rate remained substantially higher at 29.7% (95% CI 19.0–41.2) vs 5.4% (95% CI 0.7–18.2), respectively.
Treatment was well tolerated. Discontinuation rates due to adverse events were low: 1.3% in the camizestrant group and 1.9% in the AI group. The safety profile of camizestrant remained consistent with previous trials and with known profiles of CDK4/6 inhibitors.
Key Takeaways
- Camizestrant + CDK4/6i nearly doubled median PFS compared to continuing AI + CDK4/6i (16.0 vs 9.2 months).
- ctDNA surveillance enabled earlier intervention—detecting ESR1 mutations before clinical progression.
- PFS benefit was durable, with a threefold increase in 24-month PFS rate (29.7% vs 5.4%).
- PFS2 outcomes support longer-term benefit from early therapeutic switch.
- Safety and tolerability were favorable, with low rates of discontinuation.
- SERENA-6 is the first Phase 3 trial to validate ctDNA as a clinical decision-making tool in breast cancer.
The SERENA-6 trial results not only position camizestrant as a promising agent in managing endocrine-resistant breast cancer but also establish a new standard for real-time molecular monitoring. By intervening at the molecular level before visible disease progression, clinicians can now personalize treatment in a way that meaningfully extends progression-free survival and sets a new precedent in the management of HR+/HER2– ABC.
You Can Read The Full Article in NEJM
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
