AstraZeneca’s latest findings from the Phase III SERENA-6 trial highlight the potential of camizestrant, a next-generation oral selective estrogen receptor degrader (SERD), in combination with CDK4/6 inhibitors for improving first-line (1L) treatment outcomes in hormone receptor (HR)-positive, HER2-negative advanced breast cancer. The trial utilized a circulating tumor DNA (ctDNA)-guided approach to detect emergent ESR1 mutations, demonstrating that early intervention with camizestrant significantly prolongs progression-free survival (PFS) compared to standard-of-care aromatase inhibitor (AI) therapy. These results position camizestrant as a potential new standard-of-care endocrine therapy backbone, addressing endocrine resistance and reshaping the treatment paradigm for metastatic HR-positive breast cancer.
What drug is Camizestrant?
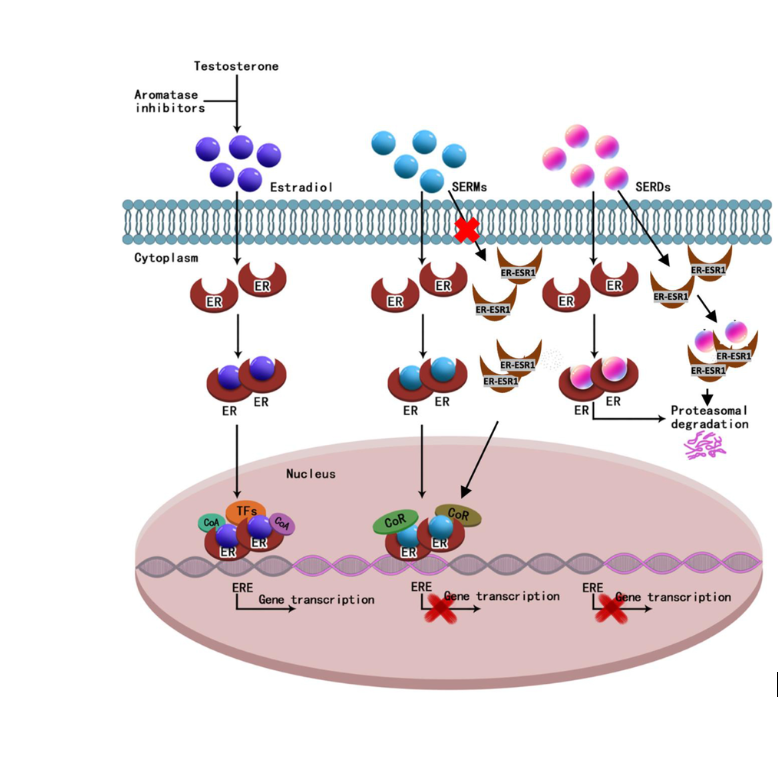
Camizestrant is a next-generation oral selective estrogen receptor degrader (SERD) and complete estrogen receptor (ER) antagonist, which is being investigated to address endocrine resistance and improve treatment outcomes.
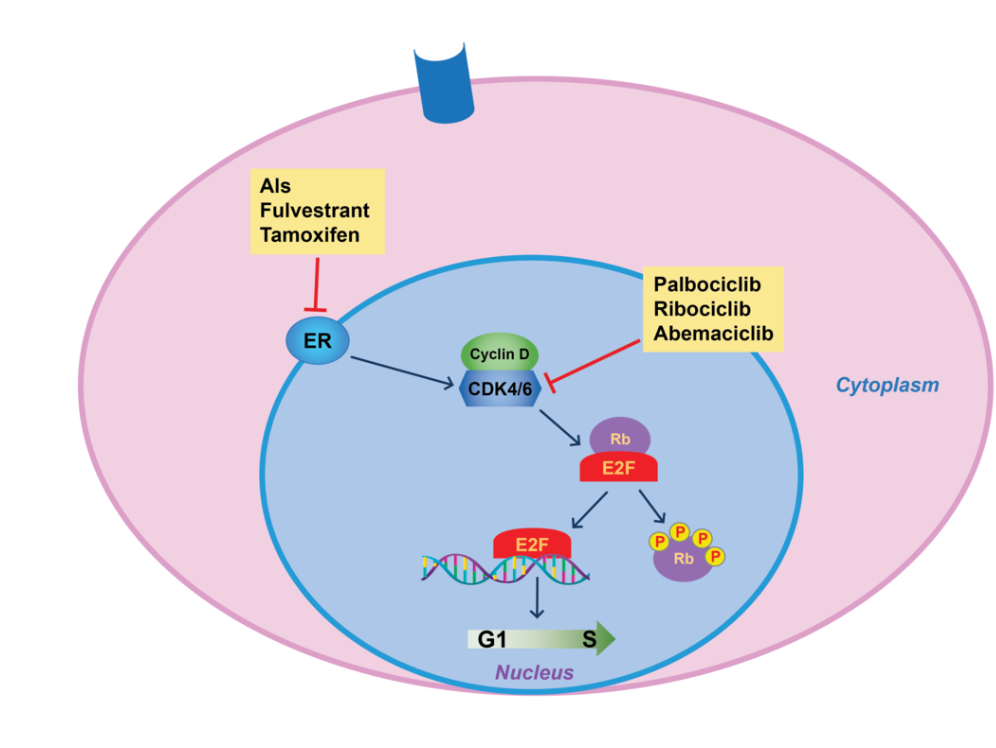
Camizestrant binds to the ligand-binding domain of the estrogen receptor (ER) with high affinity. This induces a conformational change, promoting ER degradation via the ubiquitin-proteasome pathway and by degrading ER, camizestrant reduces estrogen-driven signaling, inhibiting tumor growth. Unlike ER antagonists, camizestrant does not have proestrogenic effect in some tissues, it fully inhibits ER activity, preventing estrogen from stimulating cancer cell proliferation.This effect is particularly relevant in ESR1-mutant tumors, where ER remains constitutively active even in the absence of estrogen.
SERENA-6 trial: Methods and Design
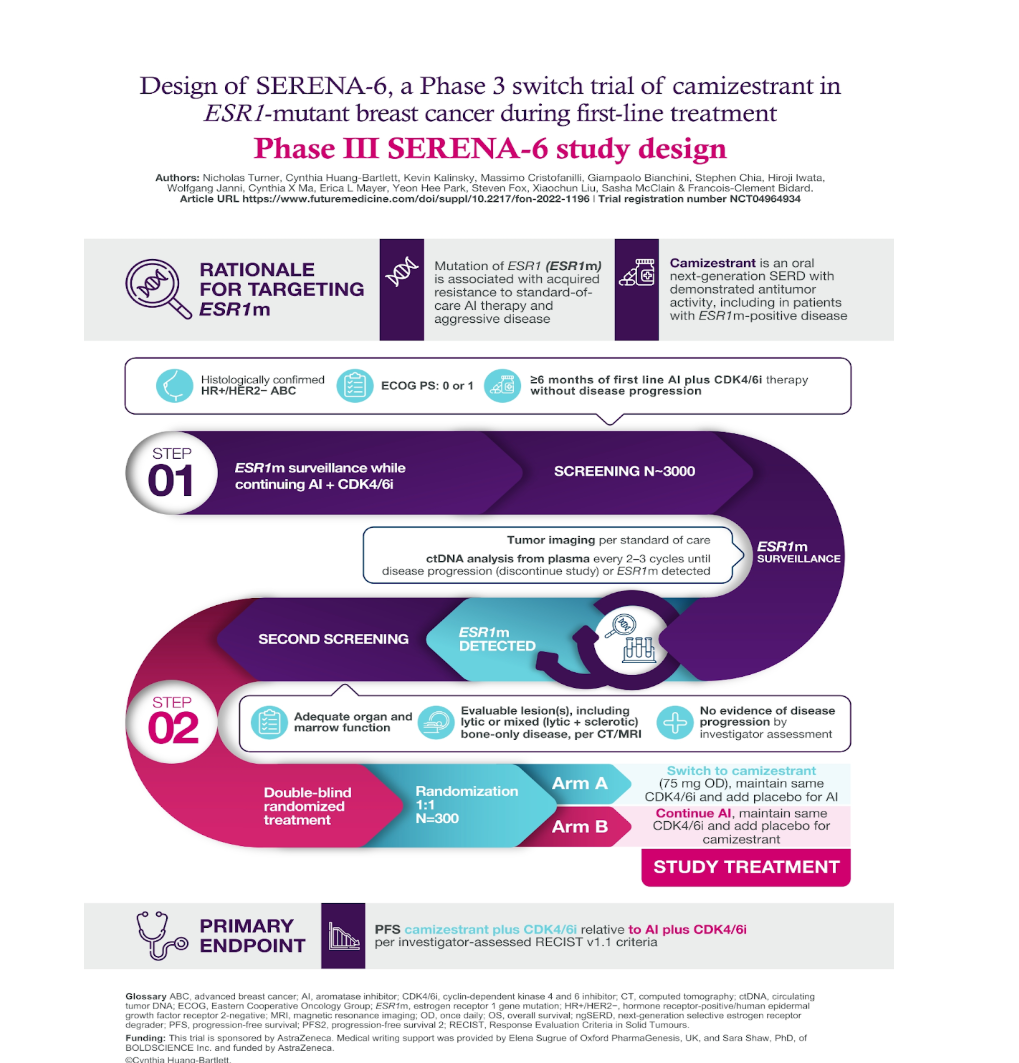
SERENA-6 is a global, double-blind, Phase III trial utilizing a circulating tumor DNA (ctDNA)-guided approach to detect emergent ESR1 mutations before clinical disease progression. The study enrolled 315 patients with HR-positive, HER2-negative advanced breast cancer receiving AI + CDK4/6 inhibitors as 1L therapy. Upon detection of an ESR1 mutation, patients were randomized to either:
- Switch to camizestrant + CDK4/6 inhibitor
- Continue AI + CDK4/6 inhibitor (standard-of-care)
The primary endpoint was progression-free survival (PFS), with key secondary endpoints including time to second disease progression (PFS2) and overall survival (OS).
Results of SERENA-6 trial
Using Camizestrant with CDK4/6 inhibitors has the potential to significantly improve outcomes in metastatic HR+ Her- breast cancers, harboring ESR1 mutations.
- Camizestrant + CDK4/6 inhibitor significantly improved PFS compared to AI + CDK4/6 inhibitor.
- PFS2 and OS data were immature at interim analysis but showed a trend toward improvement.
- Safety was consistent with known profiles of camizestrant and CDK4/6 inhibitors, with low discontinuation rates.
Key Findings
SERENA-6 trial latest updates showed benefit of using ctDNA, using new generation SERDs with CDK4/6inhibtors and more.
- First and only next-generation oral SERD to demonstrate a 1L benefit with CDK4/6 inhibitors.
- Potential paradigm shift in HR-positive, HER2-negative advanced breast cancer management.
- Early ctDNA-guided switching to camizestrant may extend endocrine therapy benefits.
Key Takeaway Messages
- Camizestrant in combination with palbociclib, ribociclib, or abemaciclib significantly delays disease progression in patients with emergent ESR1 mutations.
- ctDNA monitoring allows proactive therapeutic adjustments before clinical progression.
- Camizestrant could become a new standard-of-care endocrine therapy backbone.
What is the difference between Camizestrant vs. Elacestrant?
Elacestrant and camizestrant, both oral selective estrogen receptor degraders (SERDs), used in HR-positive, HER2-negative breast cancer treatment, differ significantly in their development stages and clinical profiles. Elacestrant, FDA-approved in January 2023, is indicated for ER-positive, HER2-negative, ESR1-mutated advanced breast cancer, with a recommended dosage of 345 mg once daily. It demonstrates a 12-month progression-free survival improvement over standard therapy. Conversely, camizestrant, still in Phase III trials, has shown promising results across broader patient populations, including those with and without ESR1 mutations. At 75 mg and 150 mg doses, camizestrant reduced disease progression risk by 42% and 33% respectively compared to fulvestrant. Their side effect profiles also differ: elacestrant commonly causes musculoskeletal pain and fatigue, while camizestrant’s distinct adverse events include photopsia and bradycardia. These differences highlight the evolving landscape of SERD therapies in breast cancer treatment.
Methods & Study Design of EMERALD trial
EMERALD trial is a phase III, randomized, open-label, international trial, which enrolled 477 postmenopausal women and men with ER- positive/Her2 – negative advanced breast cancer, who had progressed on prior endocrine therapy (1-2 lines)with CDK4/6 inhibitor.
- Patients were randomized in 2 groups, 1st- receiving Elacestrant, and 2nd- receiving standard of care (investigator’s choice: fulvestrant, anastrozole, letrozole, or exemestane). Elacestrant was administered 400 mg daily.
- Primary Endpoint was Progression-free survival (PFS) in the overall population and in ESR1-mutant patients.
- The EMERALD trial (Elacestrant) showed a modest PFS benefit (HR 0.55 for ESR1-mutant tumors) Elacestrant significantly reduced the risk of progression or death by 30% (HR = 0.70; P = .002).
Both trials highlight the potential of oral SERDs in managing ESR1-mutated advanced breast cancer. While the EMERALD trial reported a median PFS of 3.8 months for elacestrant in ESR1-mutant patients, specific median PFS data for camizestrant from SERENA-6 are pending. However, the reported significant improvement suggests a potentially more substantial benefit.
Data on the SERENA-6 trial will be presented at an upcoming medical meeting and shared with regulatory authorities. Still, now there are several discussions being held on social platforms such as X. Sara Tolary, Paolo Tarantino, Erika Hamilton, Giampolo Bianchini, Kamal Jhaveri all mentioned importance of ctDNA approach to switching from AI to camizestrant and oncology comunity is excited about SERENA-6 trial results. (Comment source LARVOL)



Read the full SERENA-6 trial data from AstraZeneca Here
Written by Sona Karamyan, MD