The fourth week of November brought notable advances across GI oncology, with updates spanning pancreatic, liver, colorectal, gastric, and biliary cancers. This week highlighted progress in AI-driven detection, early-stage immunotherapy, organ-preserving strategies, surgical innovation, and emerging therapeutic technologies.
Key developments included new AI benchmarks for pancreatic cancer detection, long-awaited immunotherapy data in potentially resectable HCC, expanded support for Non-Operative Management in rectal cancer, a large evidence review of robotic gastrectomy, real-world insights in biliary tract cancer, population-scale findings in Barrett’s esophagus, advances in CRC theranostics, early KRAS-targeted vaccine signals in pancreatic cancer, new MSI-H CRC educational resources, and updated perspectives on regulated necrosis in liver disease. Below is a curated selection of this week’s most important contributions in GI oncology.
Henkjan Huisman, PhD – Professor of AI-Guided Imaging, Radboudumc (Netherlands)
“PANORAMA benchmark identifies AI that surpasses radiologists in detecting pancreatic cancer.
Our PANORAMA study — now published in The Lancet Oncology — shows what becomes possible when AI is evaluated using a rigorously validated benchmark. The benchmark was built from nearly 400 Western CT scans, reviewed by 68 radiologists, governed by a Scientific Advisory Board, and endorsed by several international clinical organizations. This foundation is what makes performance comparisons meaningful.
Developers worldwide submitted more than 250 AI models. The top-performing systems:
Achieved 92% accuracy (radiologists: 88%)
Generated 38% fewer false positives
Because the benchmark is robust, we can now identify AI models that truly perform at an expert level. Early retrospective analyses suggest that the best benchmarked AI may detect subtle, earlier signs of pancreatic cancer without increasing unnecessary recalls — a cautiously encouraging signal for a disease so often diagnosed too late.While clinical implementation still requires further validation, this represents a substantive step toward earlier and more reliable diagnostics.
Proud of our international team, especially first authors Natalia Alves and Megan Schuurmans, and co-lead John Hermans. Funded by Horizon Europe, with follow-up work supported by Stichting Hanarth Fonds.”
Chiun Hsu, MD – Associate Dean, National Taiwan University College of Medicine (Taiwan)
“Finally in press: the clinical trial of nivolumab + ipilimumab for patients with potentially resectable hepatocellular carcinoma, sponsored by the Taiwan Cooperative Oncology Group (TCOG).
It has taken more than eight years from conceptualization to reach this point. We sincerely thank the patients and their families, the investigators across all participating institutes, and the dedicated TCOG staff members who made this possible.
Funding support from Bristol Myers Squibb and Ono Pharmaceutical is deeply appreciated.”
You can also read about Opdivo (Nivolumab): Uses in Cancer, Side Effects, Dosages, Expectations, and More on OncoDaily.
Salvatore Siena, MD – Full Professor of Medical Oncology, Università degli Studi di Milano; Principal Investigator, Ospedale Niguarda di Milano (Italy)
“The NO-CUT clinical trial, published this month in The Lancet Oncology, shows that 1 in 4 patients with microsatellite-stable locally advanced rectal cancer achieves a clinical complete response after total neoadjuvant therapy — and can avoid rectal surgery without increasing the risk of metastatic relapse.
This strategy, known as Non-Operative Management, is now a real therapeutic option in clinical practice.This Italian study, which I coordinated at Ospedale Niguarda di Milano and Università degli Studi di Milano (La Statale), enrolled 180 patients across four clinical centers:
• Ospedale Niguarda di Milano (PI Salvatore Siena)
• IEO Istituto Europeo di Oncologia (PI Maria Giulia Zampino)
• Istituto Oncologico Veneto IOV – IRCCS (PI Francesca Bergamo)
• Ospedale Papa Giovanni XXIII (PI Stefania Mosconi)The translational research program involved IFOM, Istituto Mario Negri, Candiolo Cancer Institute IRCCS, and Università degli Studi di Torino, integrating liquid biopsy and multi-omic analyses.”
Riadh Salem, MD – Surgical Registrar & DPhil Student (UK)
“I’m very pleased to share our paper in BJS Open on the evidence for robotic surgery in gastric cancer.
In this meta-analysis of 90 studies involving 65,296 patients, we found very low-certainty evidence suggesting possible benefits of robotic gastrectomy over conventional surgery. Importantly, most of the current data come from non-randomised studies at moderate risk of bias.
Our meta-regression also identified industry involvement as a significant source of heterogeneity!!
Taken together, the existing evidence base is weak. We urgently need high-quality, independent randomized controlled trials and tighter control of industry influence when generating early data on emerging surgical technologies.”
Arndt Vogel, MD – Professor of Gastrointestinal Oncology, Hannover Medical School (Germany)
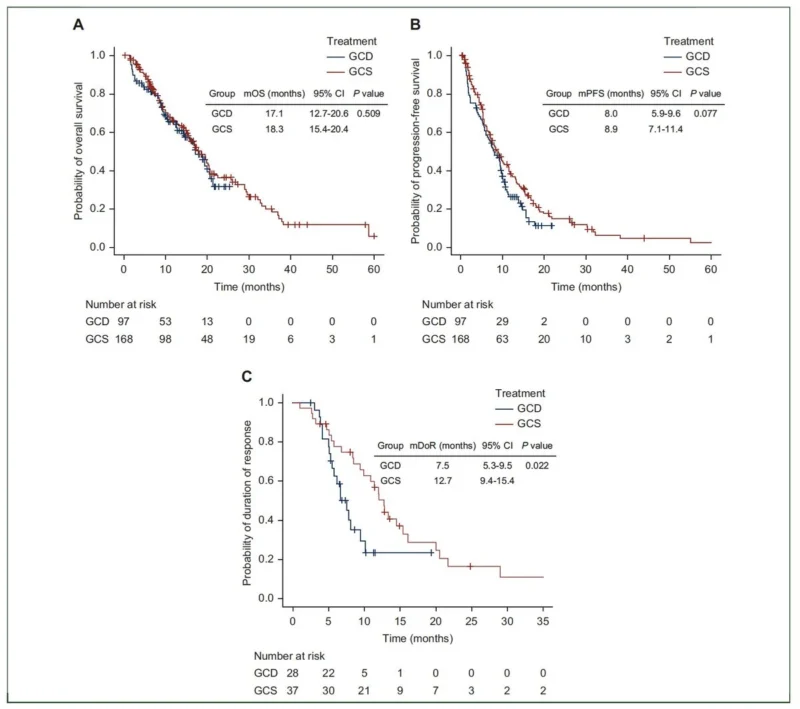
“Durvalumab + gemcitabine–cisplatin versus S-1 + gemcitabine–cisplatin in advanced biliary tract cancer — a new comparative analysis now published in ESMO Open.
Real-world dataset: 265 patients
Median OS: 17.1 vs 18.3 months
Median PFS: 8.0 vs 8.9 months
Conclusion: Comparable survival outcomes and safety in routine clinical practice.
Ismail Elkhattib, MD – Transplant Hepatology, University of Nebraska Medical Center (USA)
“New study alert!
Excited to share our latest research: In a nationwide TriNetX cohort of more than 176,000 patients with Barrett’s esophagus, combining aspirin + PPI therapy was associated with a lower risk of esophageal cancer compared with PPI alone (OR 0.799, 95% CI 0.679–0.941).
Both low-dose and high-dose aspirin showed benefit.
Is it time to add aspirin to the Barrett’s treatment regimen?Grateful for the mentorship of Dr. Houman Rezaizadeh and Dr. Khaled Elfert, and for the hard work of our co-authors Mohamed Abuelazm, Mohamed Elnaggar, and Ameer Awashra, MD.”
Sarath Chandran Chandrashekar Shenoy, MPharm, PhD – Assistant Professor, College of Pharmaceutical Sciences, Govt. Medical College Kannur (India)
“Excited to share our latest review article, ‘Theranostics in the Management of Colorectal Cancer,’ published in DiscoverNano.
The paper highlights emerging nanotechnology-driven theranostic systems, advanced molecular imaging platforms, and evolving targeted therapeutic approaches that are reshaping how colorectal cancer is detected, monitored, and treated.
I am grateful to my wonderful co-authors for their contributions — especially the lead author Dr. Gowtham Manikath, and colleagues Thejaswini Anandan, Sakshi Maruti Kolage, Onkar Kacharu Lohakare, Akshata Sanjay Ahire, and Deepu Ravindran.”
Maham Zafar – Biotechnologist & Scientific Writer; Founder & CEO, Ilm o Hunar (Pakistan)
“Pancreatic cancer remains one of the deadliest cancers, but a new type of vaccine is offering renewed hope. In a small Phase 1 clinical trial, researchers evaluated ELI-002, a vaccine designed to train the immune system to target KRAS mutations — alterations that drive more than 90% of pancreatic cancers.
Unlike personalized mRNA vaccines, ELI-002 does not require patient-specific customization, potentially making it far more scalable. The vaccine stimulates KRAS-specific T-cell responses against cancer cells, including residual disease after surgery or chemotherapy.
In the trial:
• 21 of 25 patients developed KRAS-directed immune responses
• Relapse-free survival: ~15 months
• Overall survival: ~29 months
These outcomes exceed what is typically expected in this setting, with similarly encouraging signals observed in colorectal cancer patients.While the findings are promising, Phase 1 studies primarily assess safety, and many early successes do not translate into later-phase benefit. Larger Phase 2 and 3 trials will be essential to confirm whether ELI-002 meaningfully improves survival. Still, this work underscores the growing potential of cancer vaccines in pancreatic cancer, as highlighted by researchers at MD Anderson Cancer Center.”

Nicholas DeVito, MD – Assistant Professor of Medicine, Division of Medical Oncology, Duke University (USA)
“My MSI-H colorectal cancer management talk is now available on the Duke Cancer Institute YouTube channel!”
Tom Lüdde, MD, PhD – Director, Department of Gastroenterology, Hepatology & Infectious Diseases (Germany)
“Happy to share our new review in Nature Reviews Gastroenterology & Hepatology on ‘Regulated necrosis at the crossroads of liver inflammation and cancer development.’
In this article, we summarize how necroptosis, pyroptosis, and ferroptosis contribute to inflammatory liver diseases and hepatocellular carcinoma. We highlight ongoing controversies regarding their functions in hepatocytes versus non-parenchymal cells, and discuss emerging therapeutic strategies that target these regulated cell-death pathways to slow disease progression or enhance anti-tumor immune responses.
We also outline key methodological advances and outstanding questions that must be resolved to translate discoveries from preclinical models into meaningful clinical applications.”

You can also read about 10 Must-Read Posts in GI Oncology from the third week of November on OncoDaily.


