The ESMO Congress 2025 is a major global oncology event organized by the European Society for Medical Oncology (ESMO).
It is taking place at Messe Berlin in Berlin, Germany, from October 17 to 21, 2025. The congress features a comprehensive scientific and educational program designed to foster exchange and debate in translational cancer science, showcasing potentially practice-changing data, and stimulating multidisciplinary discussions to improve cancer treatment options.
Arndt Vogel, Head of the Visceral Oncology Center, Head of the Center for Personalized Medicine, MHH, and Senior Consultant of the Department of Gastroenterology, Hepatology and Endocrinology at Hannover Medical School, shared GI highlights from ESMO 2025 on X:
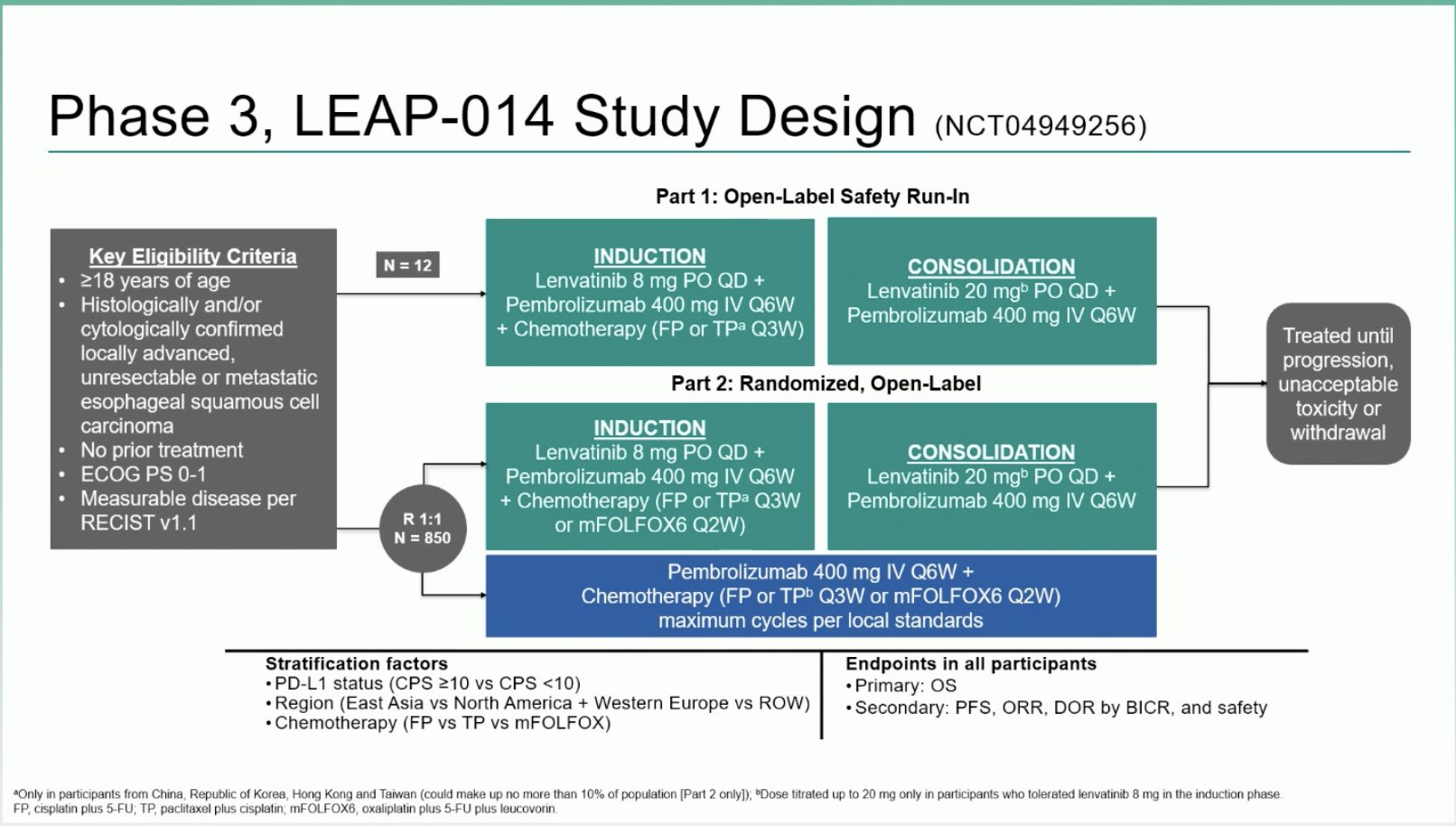
“LEAP-014: Lenvatinib plus CTx and pembrolizumab vs pembrolizumab plus CTx in metastatic ESCC
- ORR: 62 vs 54%
- mPFS: 7.2 vs 6.9 mo
- mOS: 17.6 vs 15.5 mo
- negative study, no benefit by addition of lenvatinib to CTx + Pembro, more toxicity.”
“Invited Discussant LBA81 and 2094O
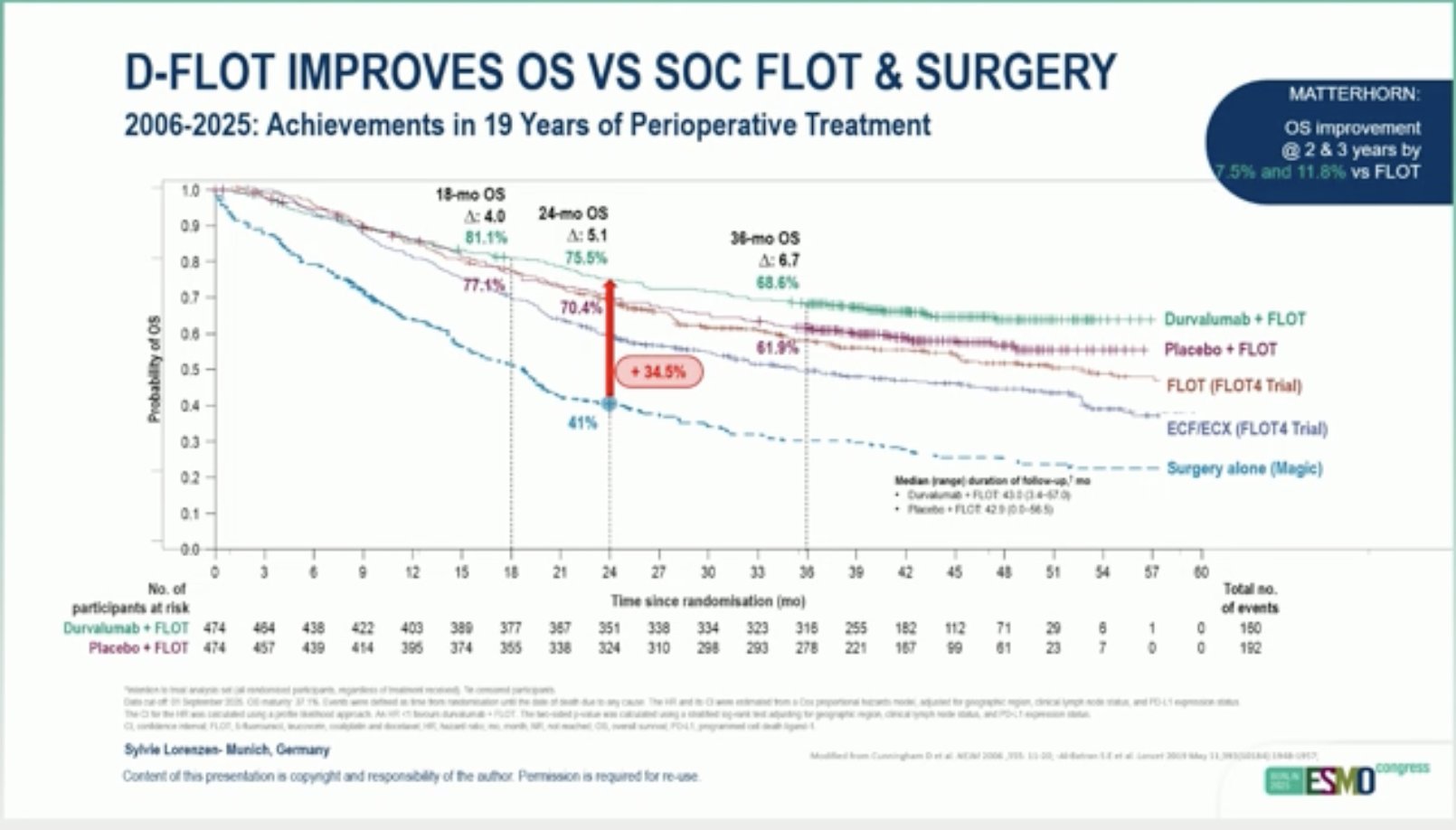
Durvalumab improves pCT and MPR in resectable G/ GEJ adenocarcinoma, with significant clinical benefit.”
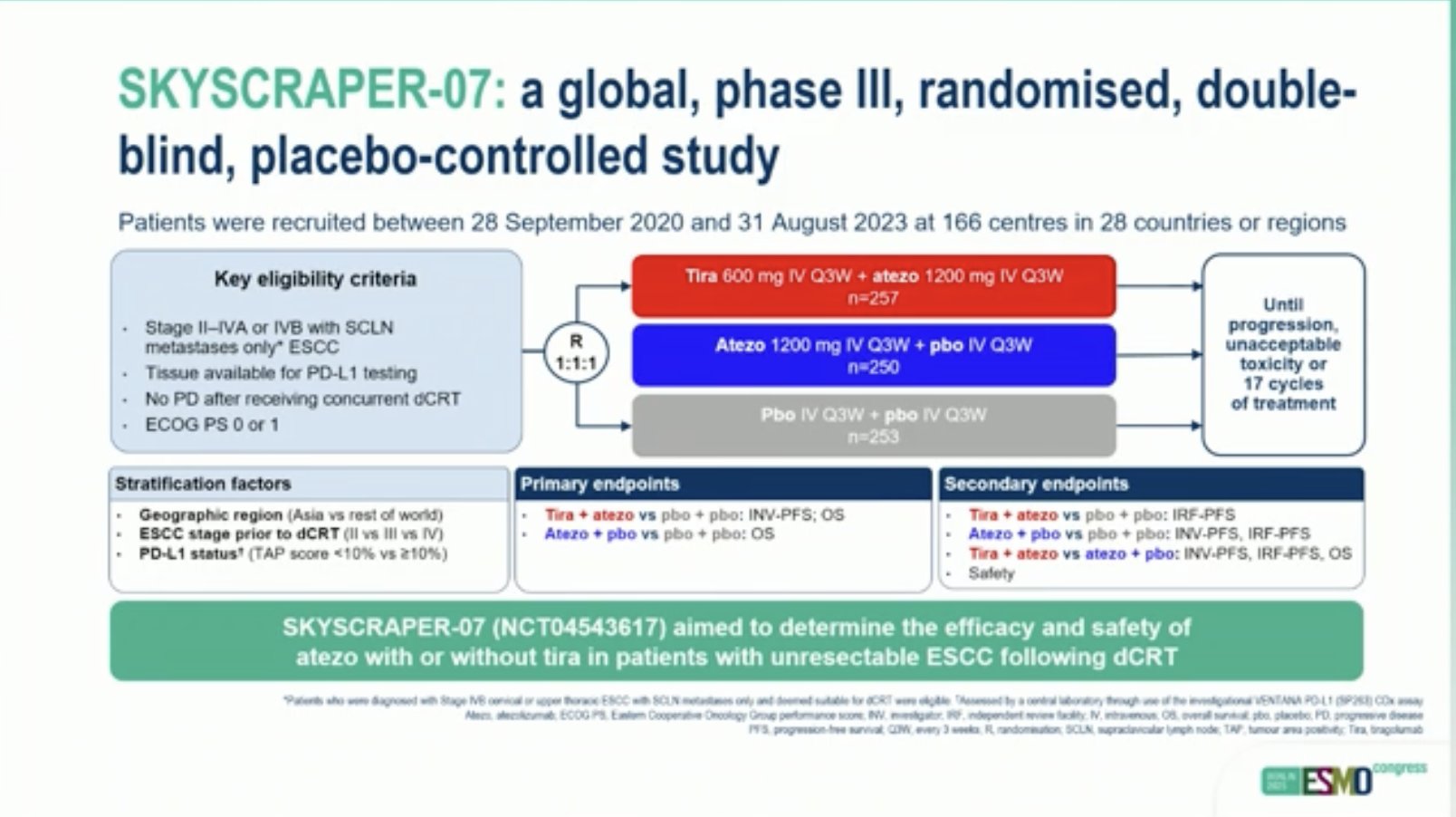
“SKYSCRAPER-07: Phase III study of atezo with or without tiragolumab in unresectable ESCC that has not progressed following dCRT
- mPFS: 29 vs 20.8 vs 16.6
- mOS: n.r. vs 38 vs 36 mo
- Atezo improves outcome, but adding tiragolumab to atezo with detrimental effect.”
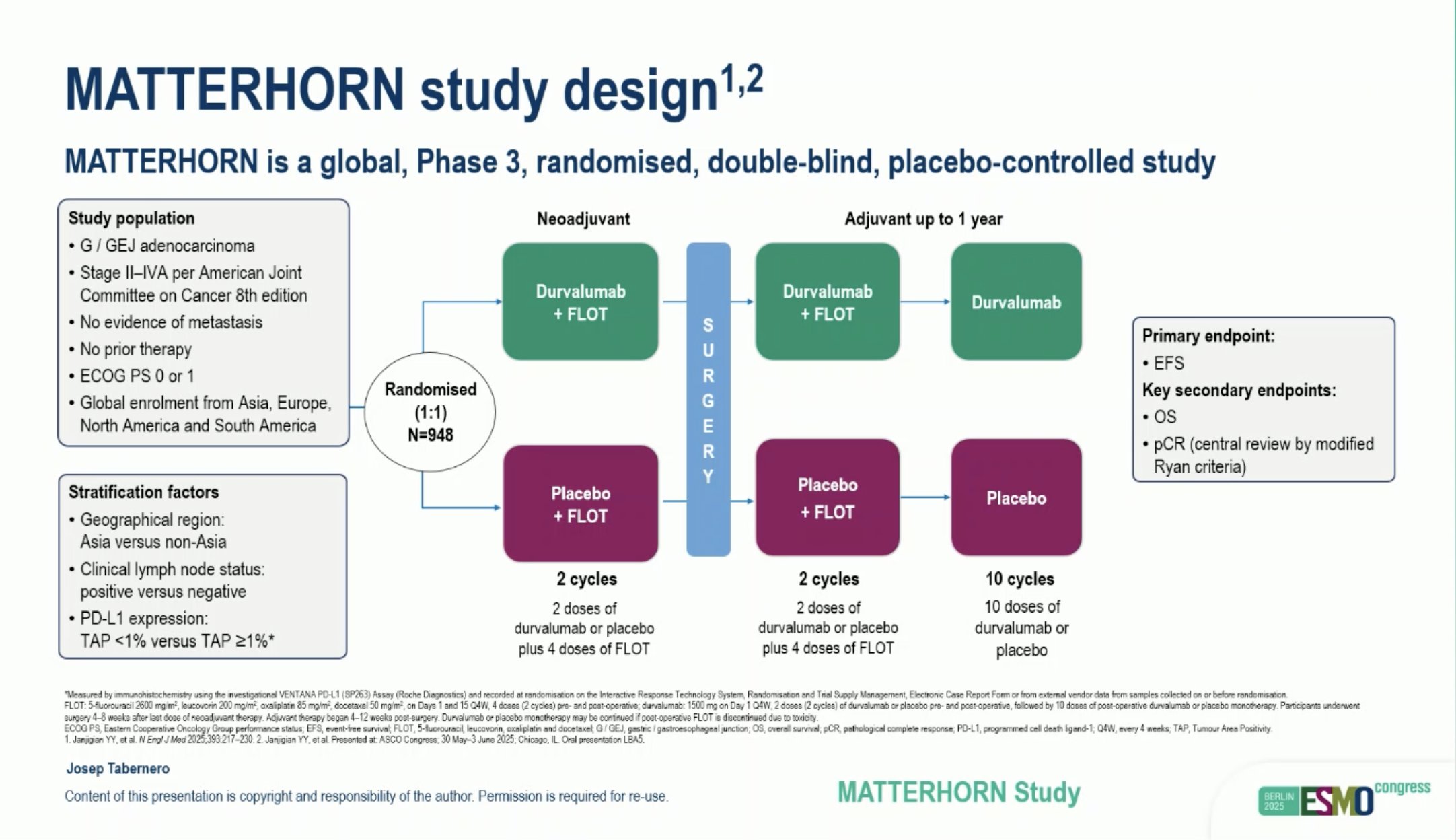
“MATTERHORN: Phase III study of durvalumab + FLOT in resectable G / GEJ adenocarcinoma
- pCR: 16%, MPR: 26%, any: 87%
- OS: HR: 0.78; 36-mo OS: 68 vs 61%
- OS improved independent of TAP, better in responders > new SOC.”
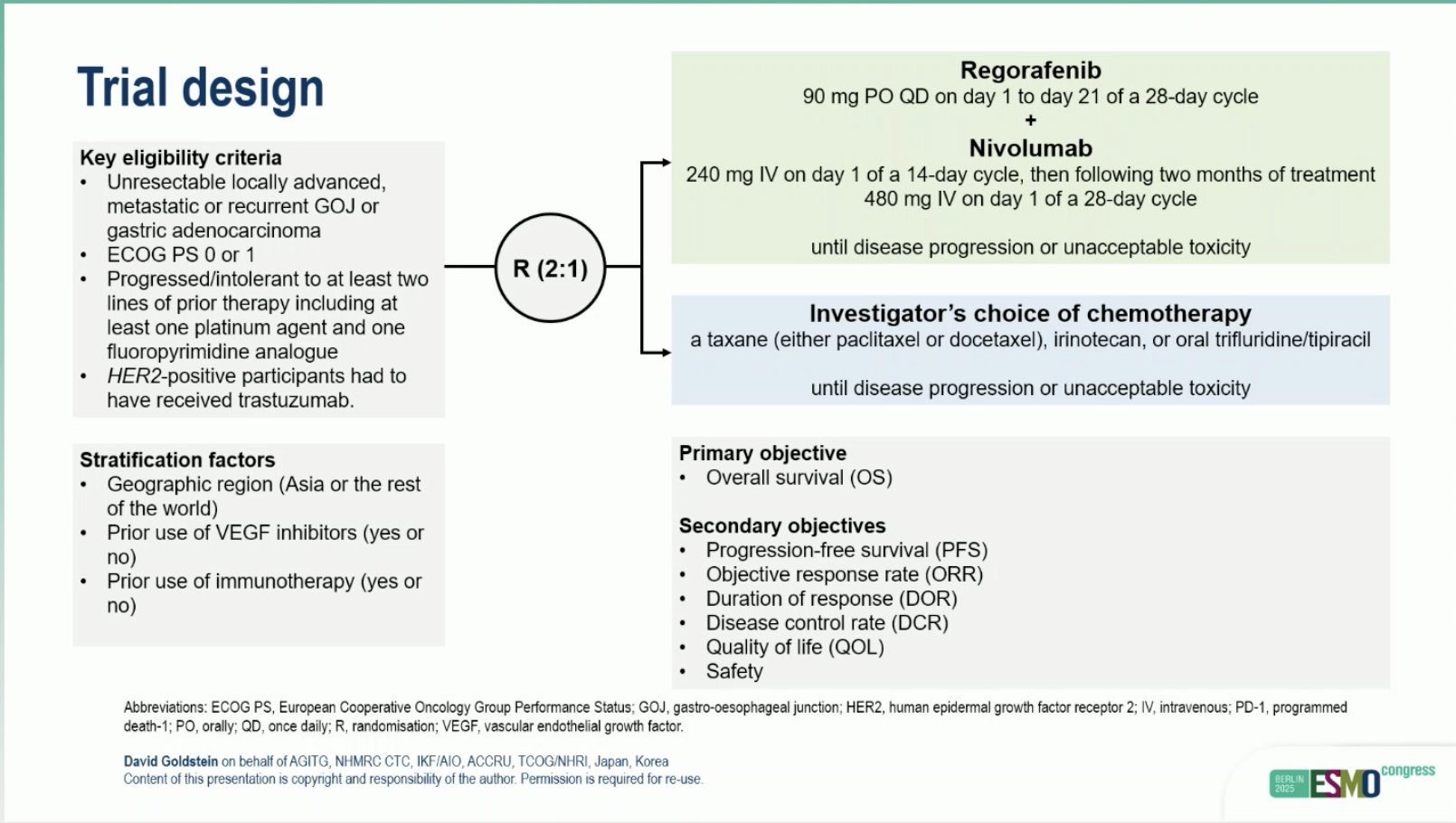
“INTEGRATE IIb: Regorafenib plus nivolumab vs investigator’s choice of CTx in previously treated G/ GEJ adenocarcinoma
- ORR: 7.4 vs 2.6%
- mPFS: 1.9 vs 1.9 mo
- mOS: 7.2 vs 6.9 mo
- study, but Rego may remain a backbone for chemo-free combinations.”
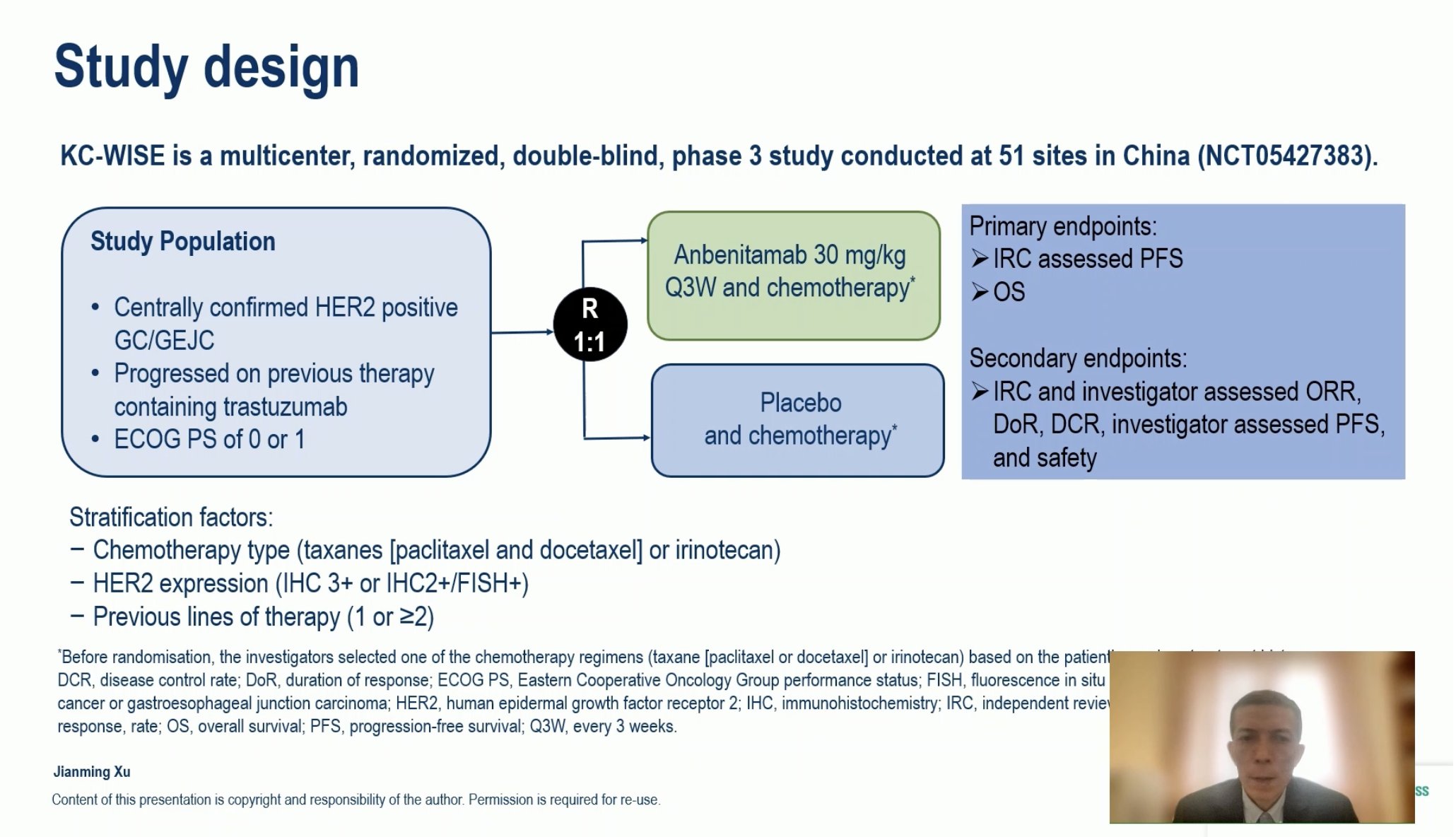
“KC-WISE: Anbenitamab plus chemotherapy for HER2-positive GC/GEJC after Trastuzumab treatment
- ORR: 55 vs 10.8%
- mPFS: 7.1 vs 2.7 mo
- mOS: 19.6 vs 11.5 mo
- Bi-specific antibody with significant benefit in HER2 pretreated patients.”
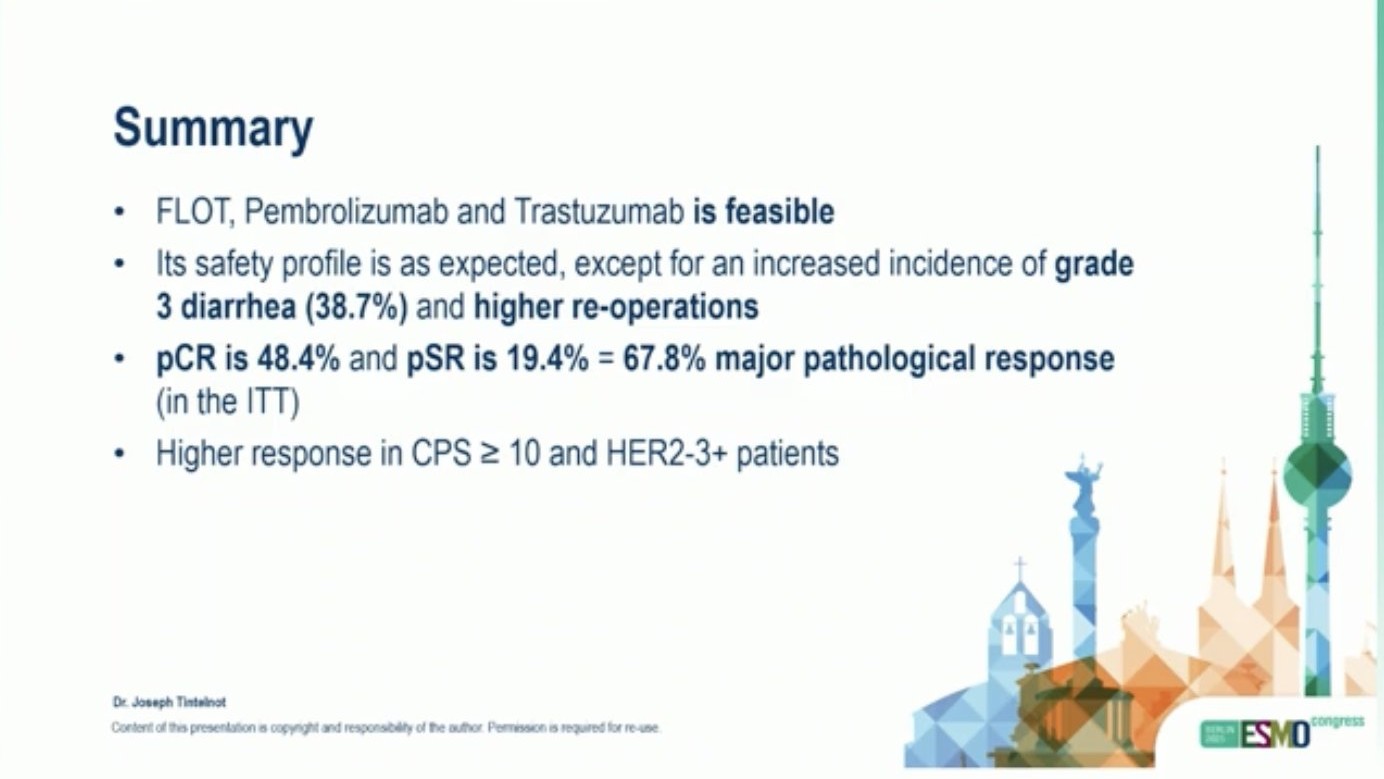
“PHERFLOT/IKF-053: Pembrolizumab and trastuzumab in combination with FLOT in the perioperative treatment of HER2+ GC7GEJ cancer
pCR: 48%,
Higher incidence gr. 3° diarrhea and more reoperations
Feasible and effective, published in Nature Medicine.”
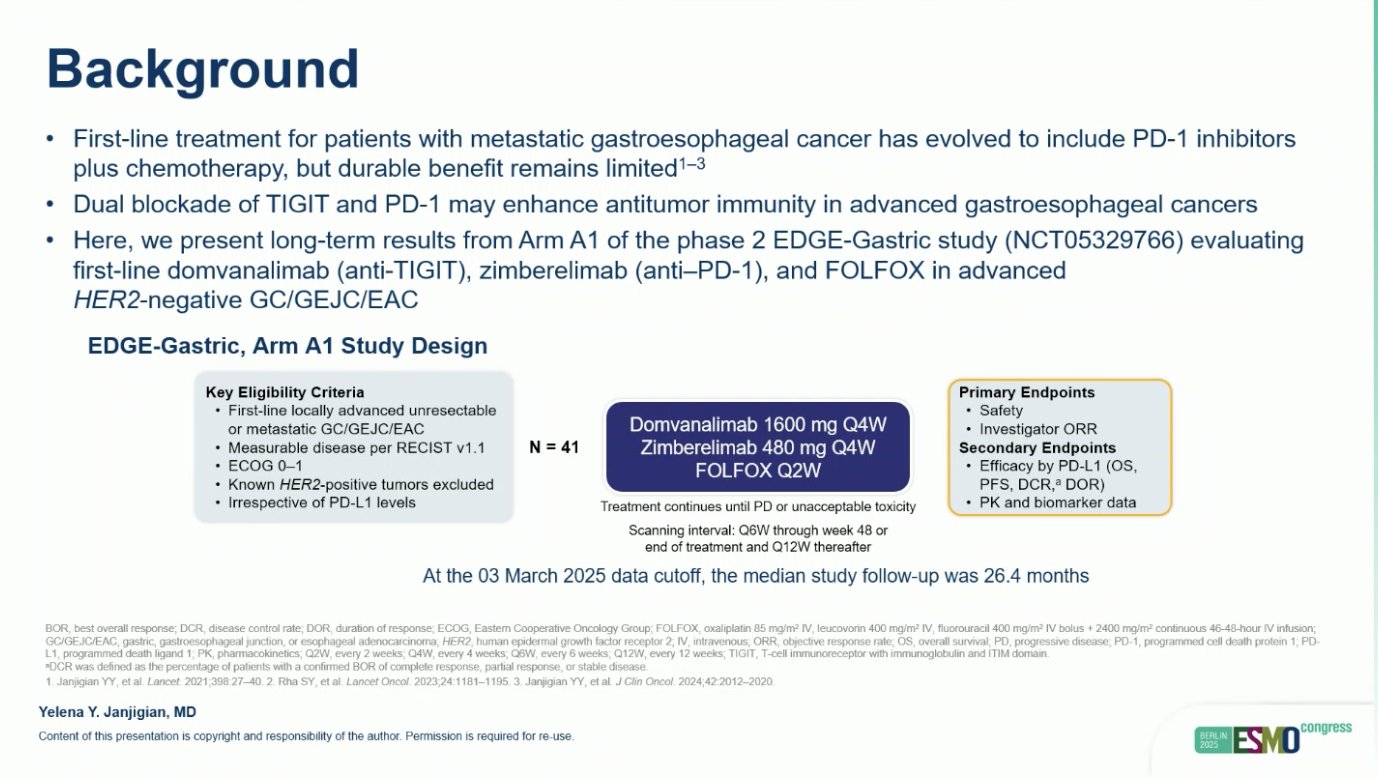
“Domvanalimab, zimberelimab, and FOLFOX in advanced GC/GEJC/EAC
- ORR: 59%, TAP>5% 69%
- mPFS: 12.9 mo
- mOS: 26.7%
- Promising efficacy for anti-TIGIT plus anti-PD1.”
“EC-CRT-002: Tislelizumab combined with induction CTR in locally advanced esophageal squamous cell carcinoma
Adding Tislelizumab improves efficacy of neoadjuvant CTR in ESCC, no benefit for adjuvant.”
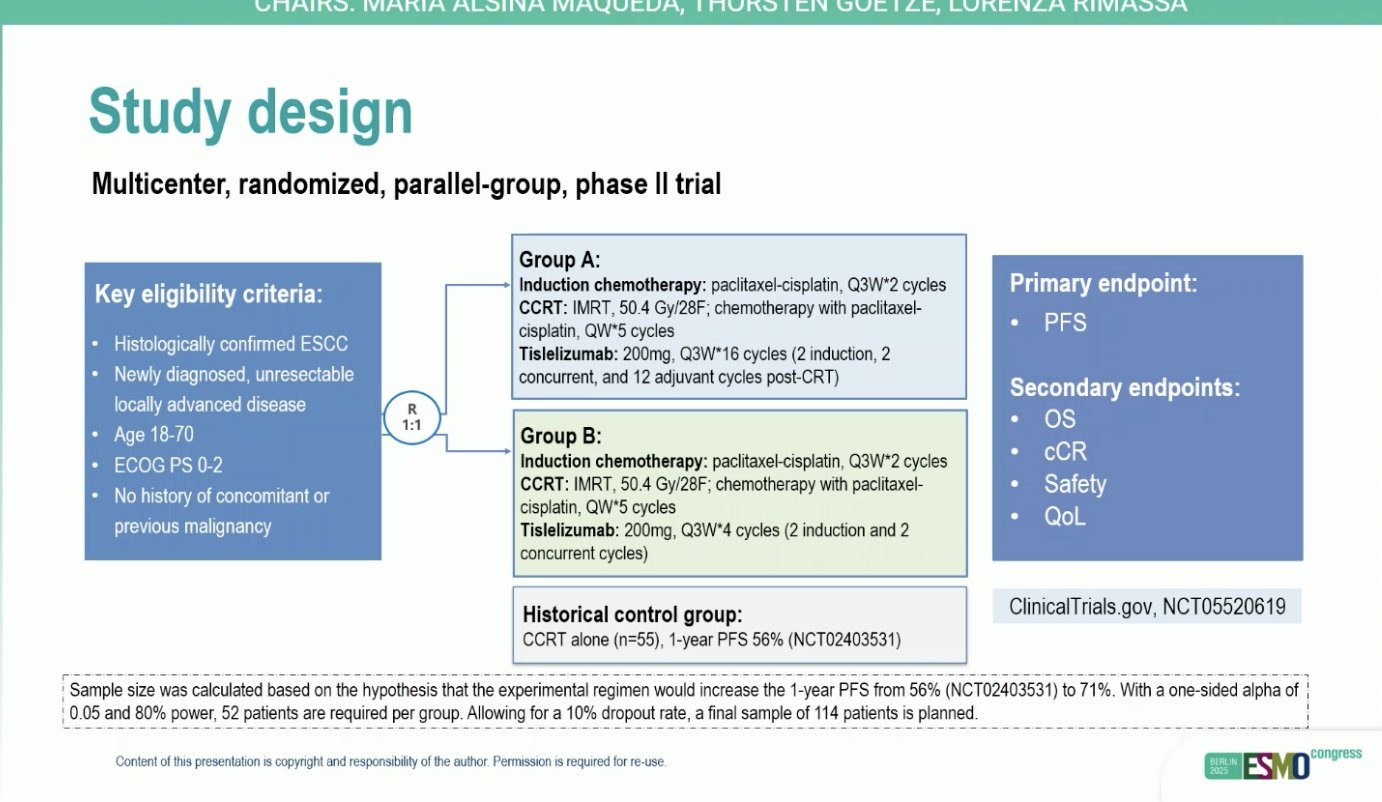
“PERISCOPE II trial: Systemic therapy, gastrectomy and CRS/HIPEC vs systemic therapy alone for gastric cancer with limited peritoneal dissemination
- mOS: 16.6 vs 15.6 mo
- no benefit for CRS/HIPEC for pts with limited peritoneal metastasis, significant toxicity.”
“COMPASSION-15: Cadonilimab plus CTx vs CTx as 1L treatment for advanced G/GEJ adenocarcinoma
- mPFS: 7 vs 5.3 mo
- mOS: 13.9 vs 11.1
- Bispecific antibody targeting PD-1 and CTL4 improves outcome specifically in CPS ≥5.”
“RC118 (CLDN18.2-ADC) combined with PD-1 blockade or RC148 (PD-1/VEGF bispecific) for locally advanced la/m G/GEJA
- ORR: 57 vs 33%, DCR: 95 vs 67%
- mPFS: 7.9 vs 4.3 mo
- Interesting efficacy for the bispecific AB in combo with anti-CLDN18.2.”
“CS1003-305 phs-3: Nofazinlimab + lenvatinib vs plc + lenvatinib as 1L treatment for HCC
- ORR: 33 vs 19%
- mPFS: 9.2 vs 6.9 mo
- mOS: 21.6 vs 18.5 mo
As in LEAP-002, trend for better outcomes, but negative trial.”
“PRODIGE 81/FFCD 2101 – TRIPLET HCC: Adding IPI to ATEZO + BEV in 1L uHCC
- ORR: 30 vs 27.4%
- mPFS: 8.0 vs 9.6 mo
- mOS: CC vs XX mo
No benefit for adding IPI, surprisingly clear negative..not enough anti-CTL4?”
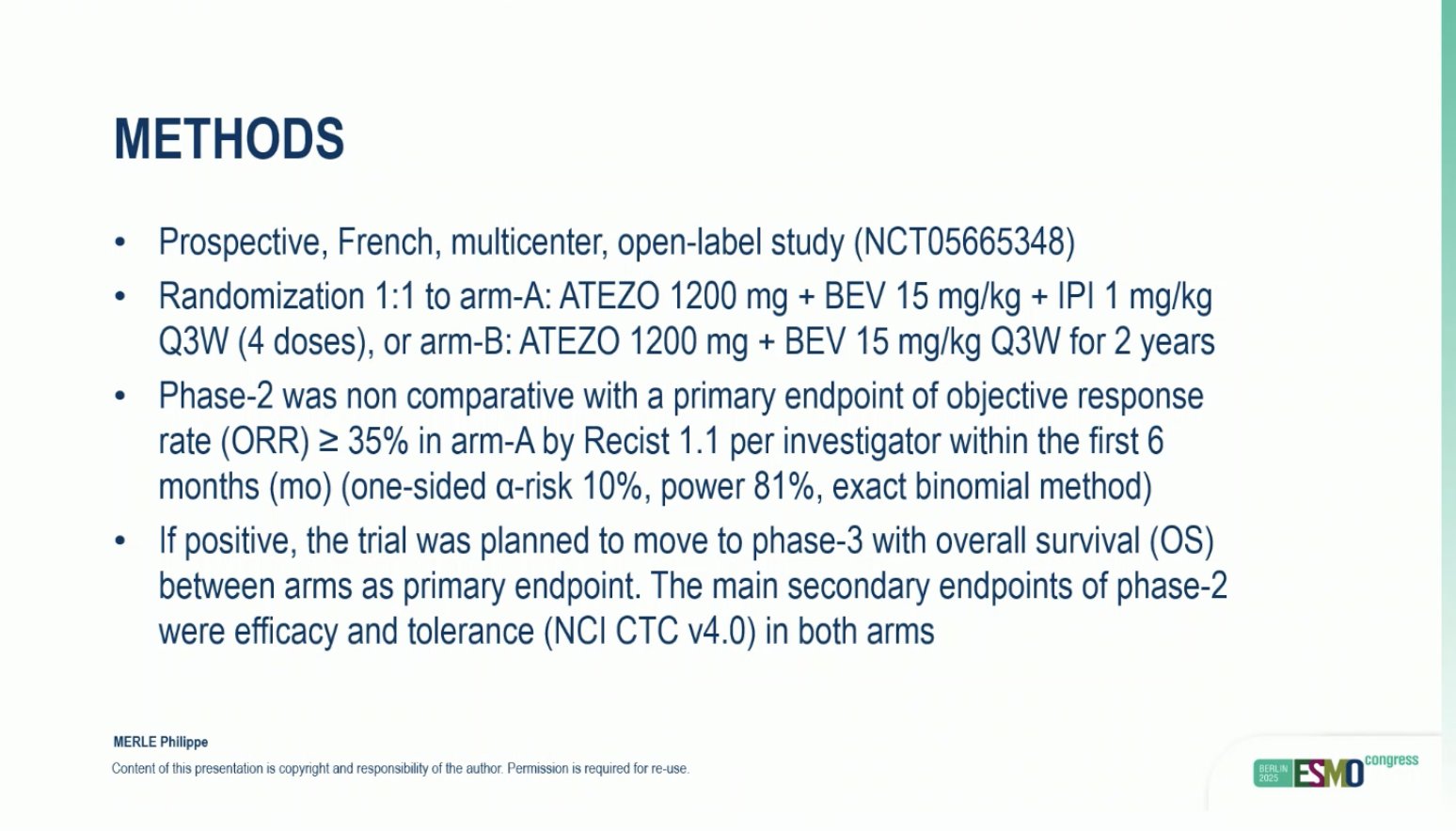
“PEGASUS trial: Post-surgical liquid biopsy-guided treatment of stage III and high-risk stage II CRC patients
- ctDNA trajectories define prognosis
- 3yr-DFS: 82 (-) vs 58% (+)
Very innovative trial design, ctDNA guided ACT is the future.”
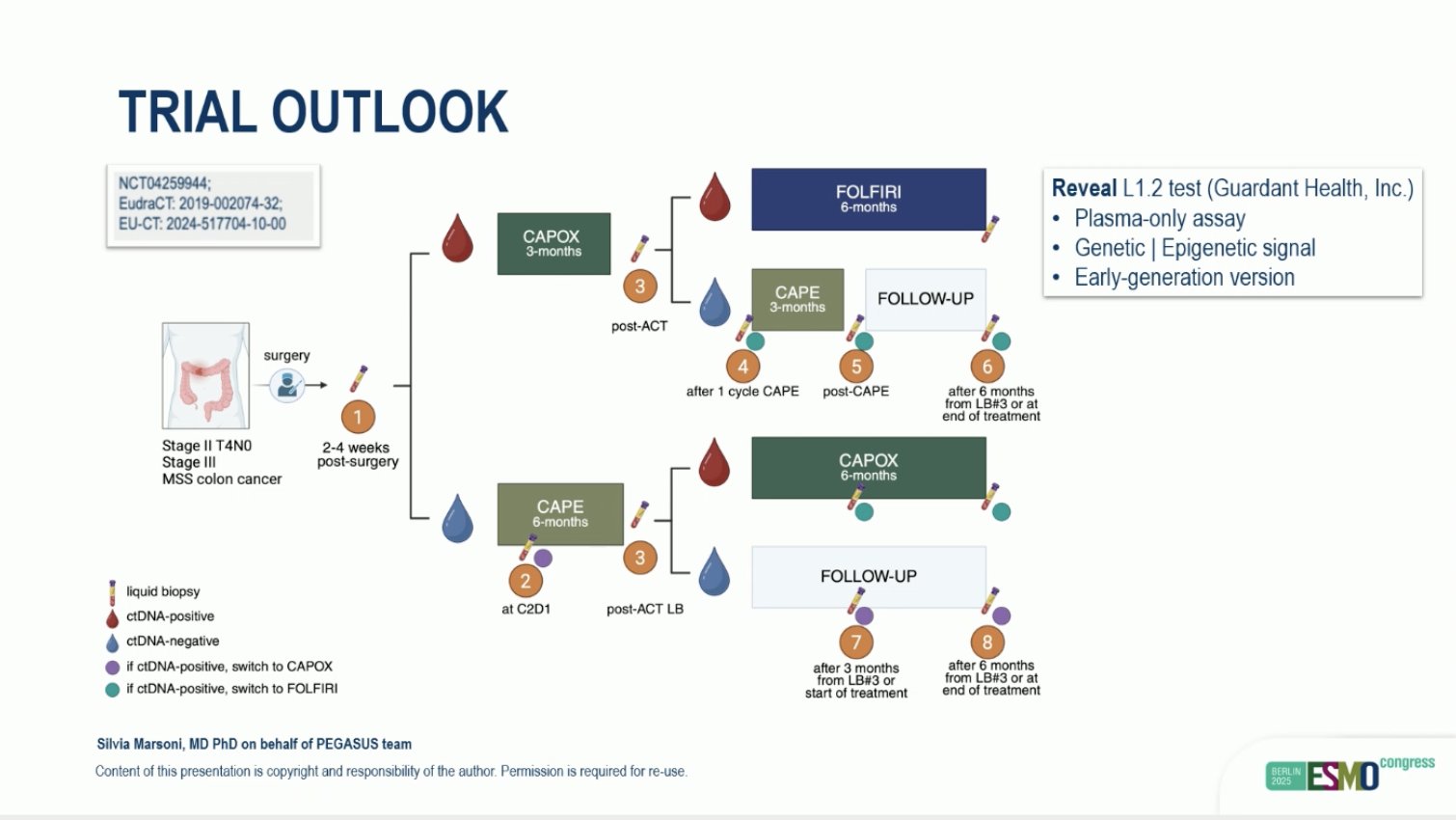
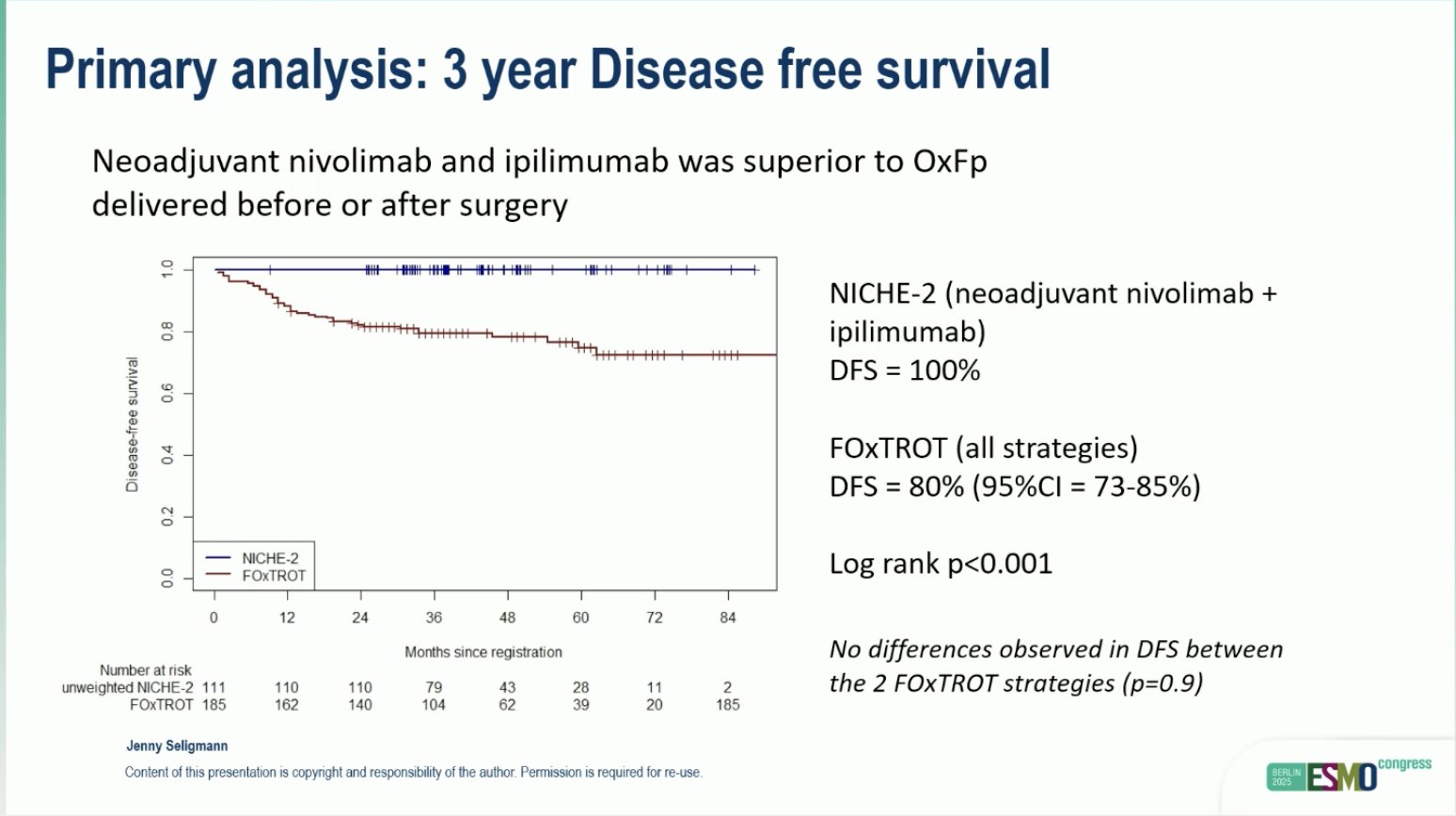
“Comparison of outcomes in clinical trials of locally advanced dMMR colon cancer: FOxTROT and NICHE-2
- patient-based comparison
- Final coffin nail for CTx
- Can we omit surgery next?
Amazing efficacy for ICI in MSI CRC.”
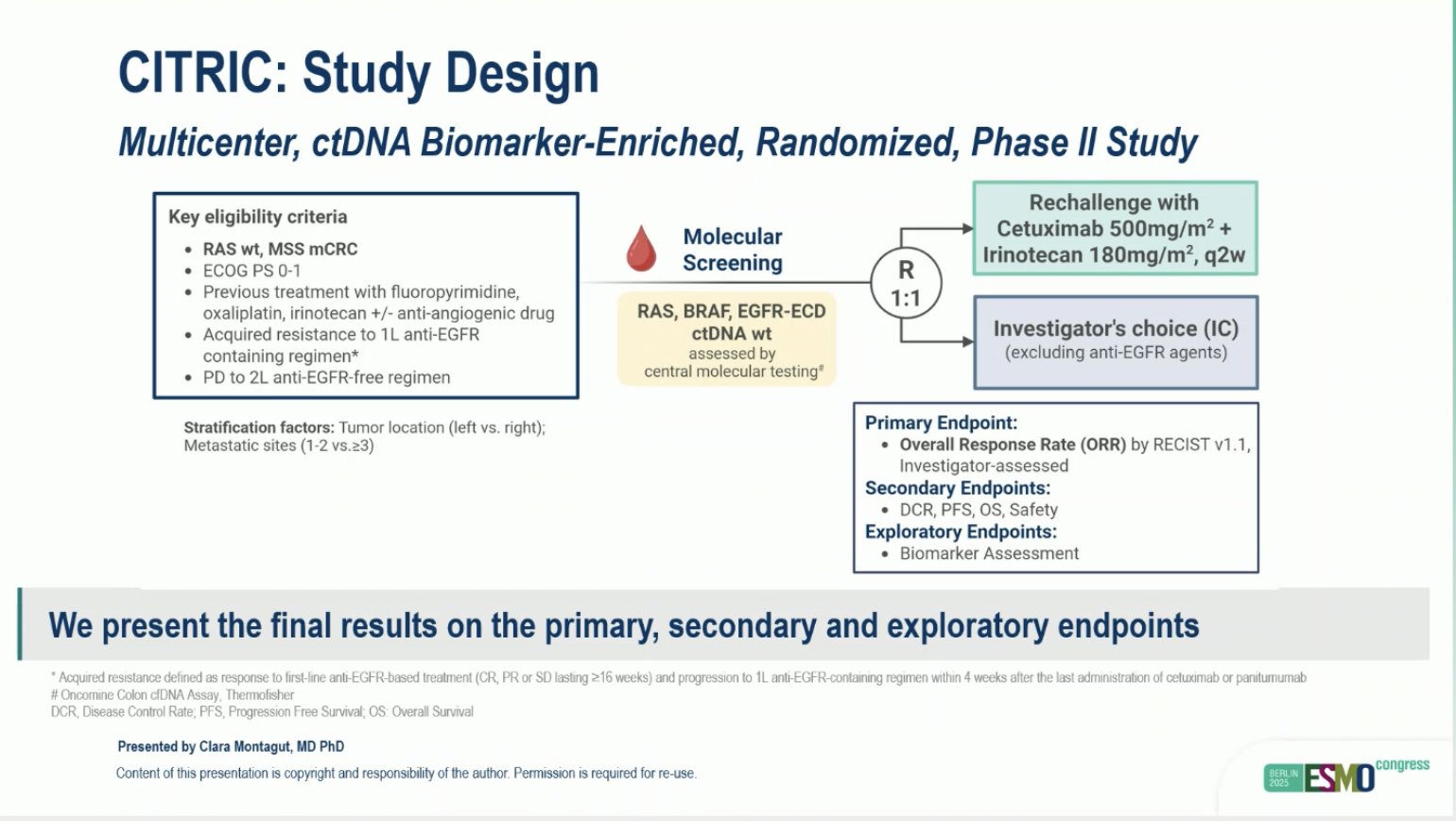
“CITRIC phs-2: ctDNA-guided anti-EGFR rechallenge strategy in mCRC
- ORR: 9.7 vs 3.7%, DCR: 77 vs 44%
- mPFS: 4.6 vs 2 mo
- mOS: 11.3 vs 7.3 mo
negative study, maybe ultraselection is required.”
“PARERE trial: Panitumumab retreatment followed by regorafenib vs the reverse sequence in chemorefractory mCRC with RAS/ BRAF WT ctDNA
- mOS: 11.6 vs 11.7mo
- Better PFS for pani in both arms
negative trial, but rechallenge is an option in 4L.”
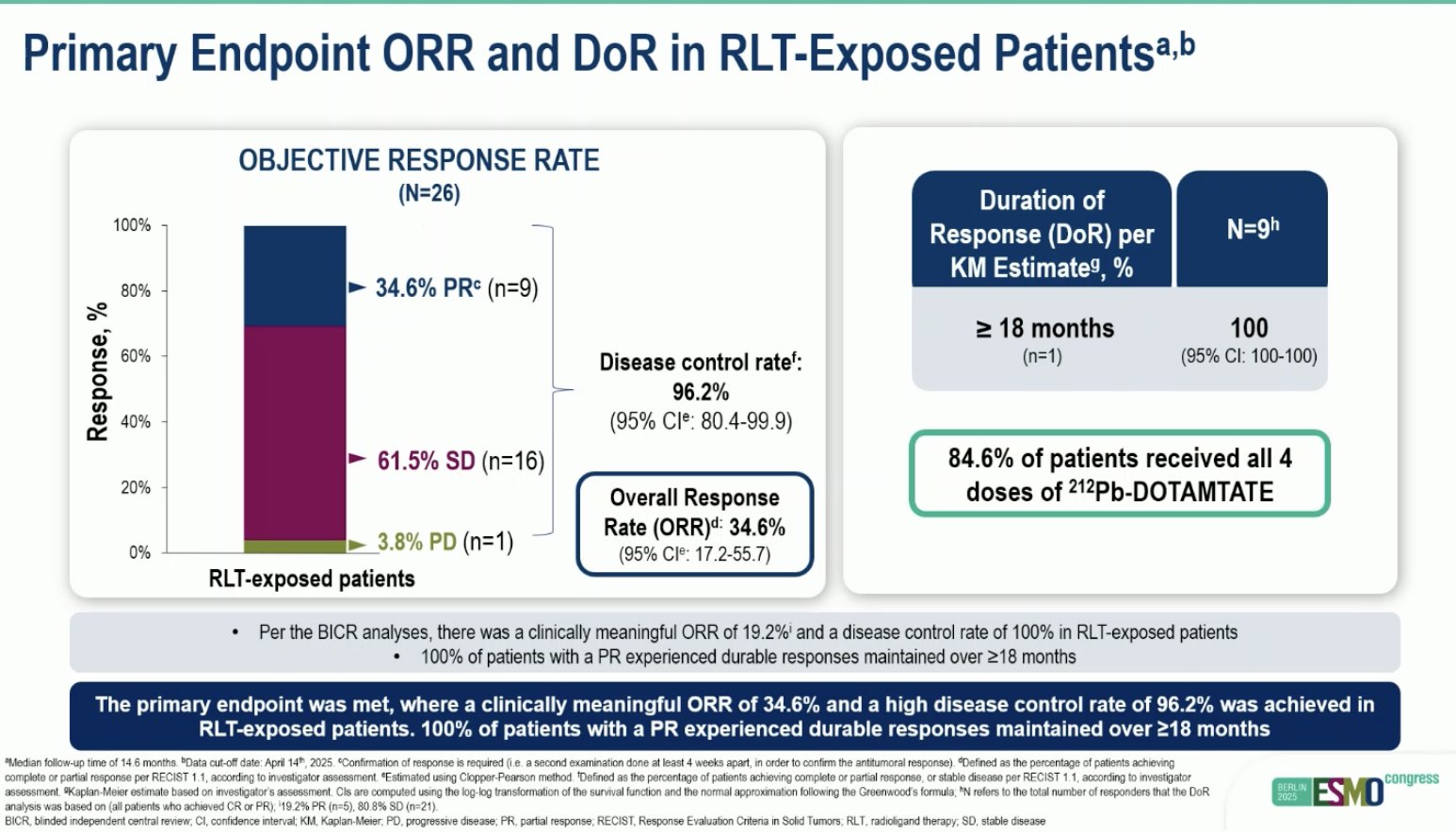
“Efficacy and safety of 212Pb-DOTAMTATE in unresectable or metastatic GEP-NET previously treated with PRRT
- ORR: 34%
- 18-mo PFS: 82%
- 18-mo OS: 81%
- Dysphagia in 57%
promising efficacy for TAT after PRRT, Dysphagia needs to be managed.”
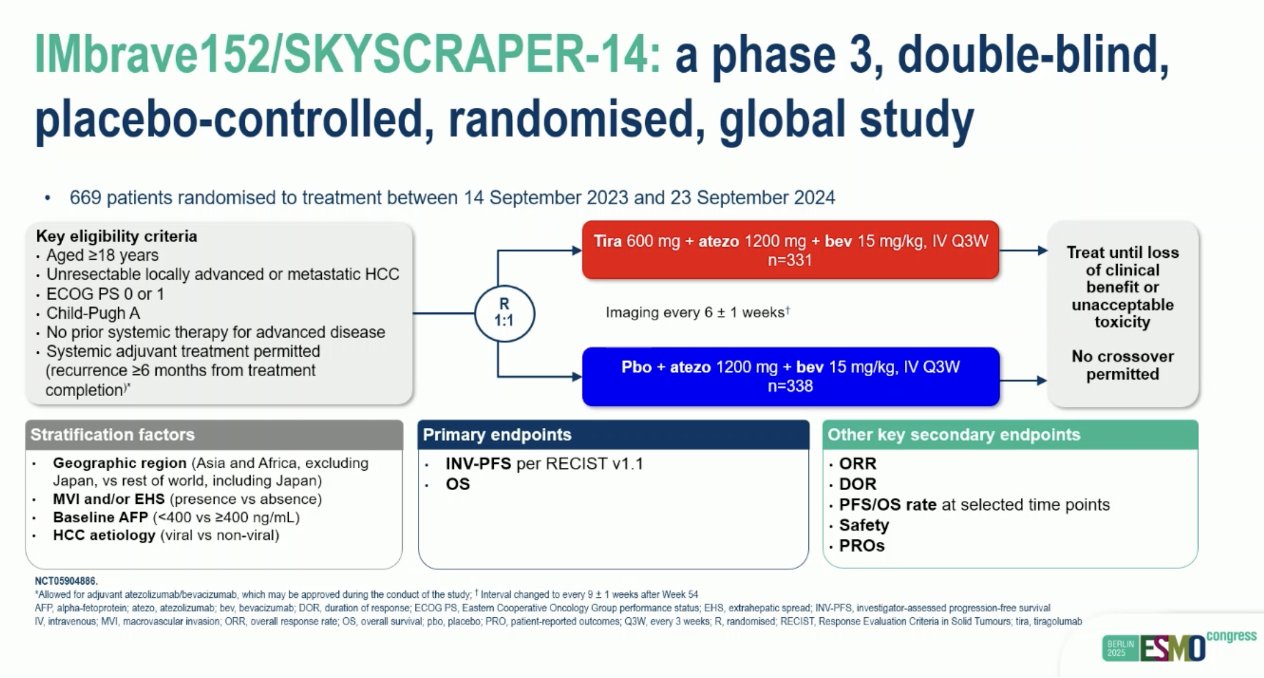
“IMbrave152/SKYSCRAPER-14 phs III: tiragolumab + atezo/bev vs pbo + atezo/bev in 1L HCC
- ORR: 30 vs 26%
- mPFS: 8.3 vs 8.2 mo
- mOS: HR: 0.94
Second neg. triplet HCC study at ESMO 2025 and another negative. Study for tira…”
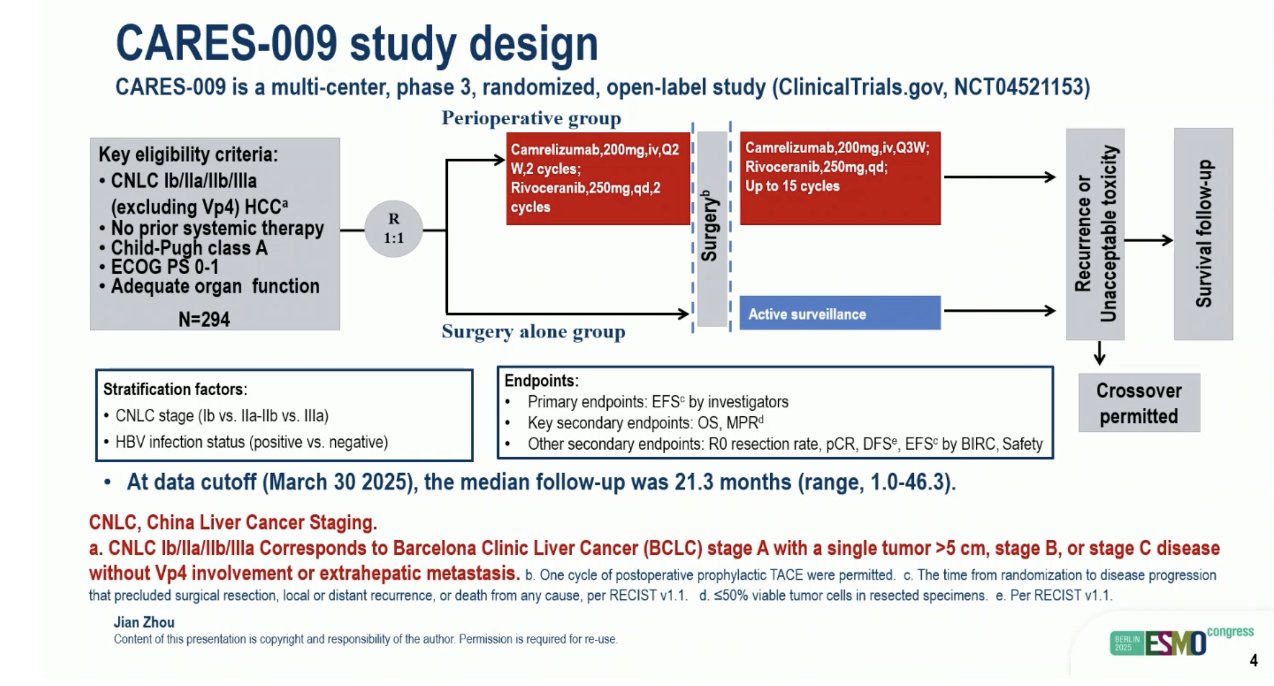
“CARES-009: Perioperative camrelizumab plus rivoceranib in resectable hepatocellular carcinoma
- EFS: 42 vs 19 mo
- DFS: 42 vs 19 mo
- MPR: 35 vs 7%
Very promising results, neoadjuvant strategies will be the future in HCC.”
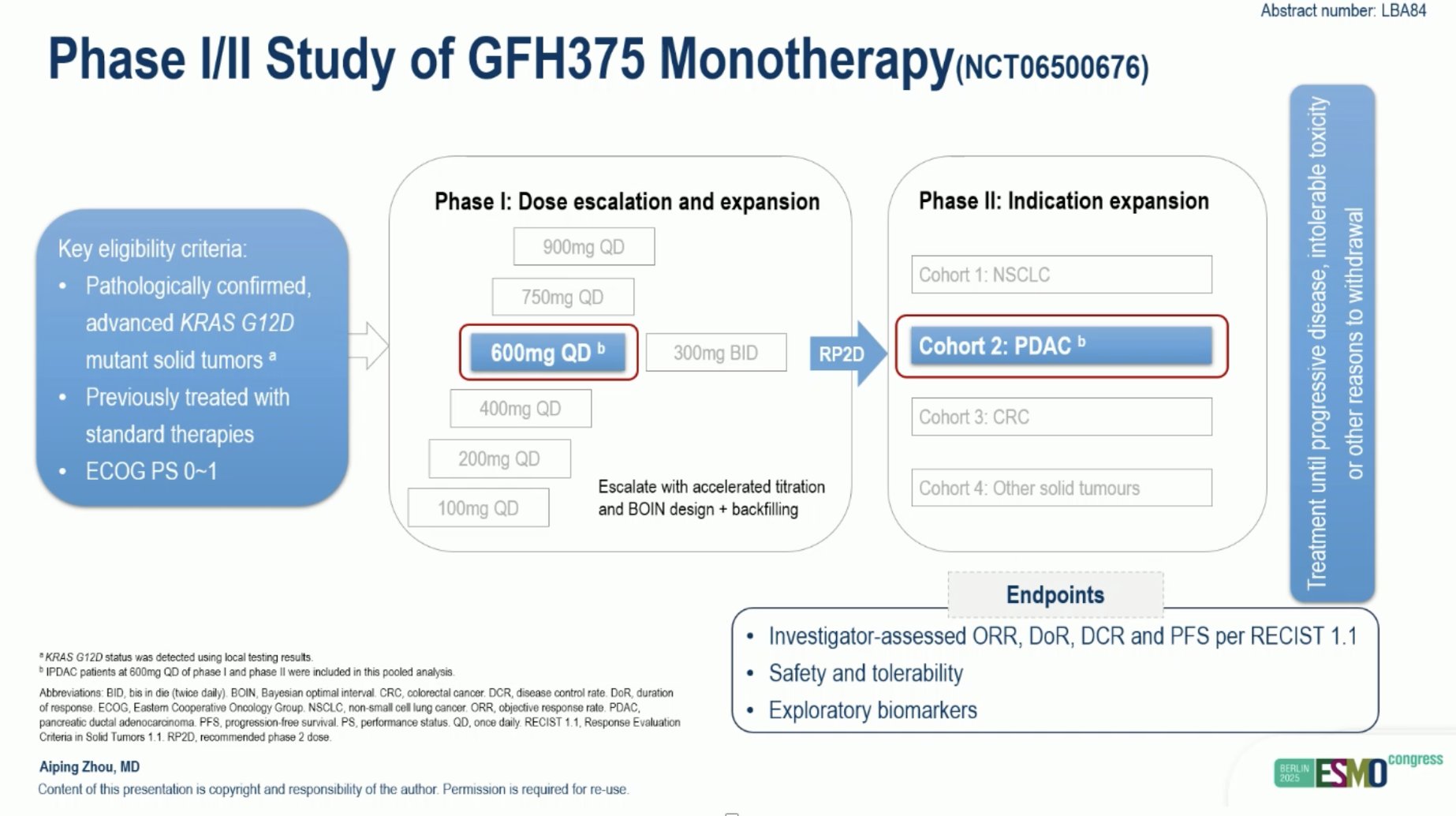
“Efficacy and safety of GFH375 monotherapy in previously treated advanced KRAS G12D PDAC
- ORR: 41%, DCR: 97%
- mPFS: 5.5 mo
- 1-yr OS rate 92%
very promising, but secondary resistance may be a concern > earlier line and combinations?”
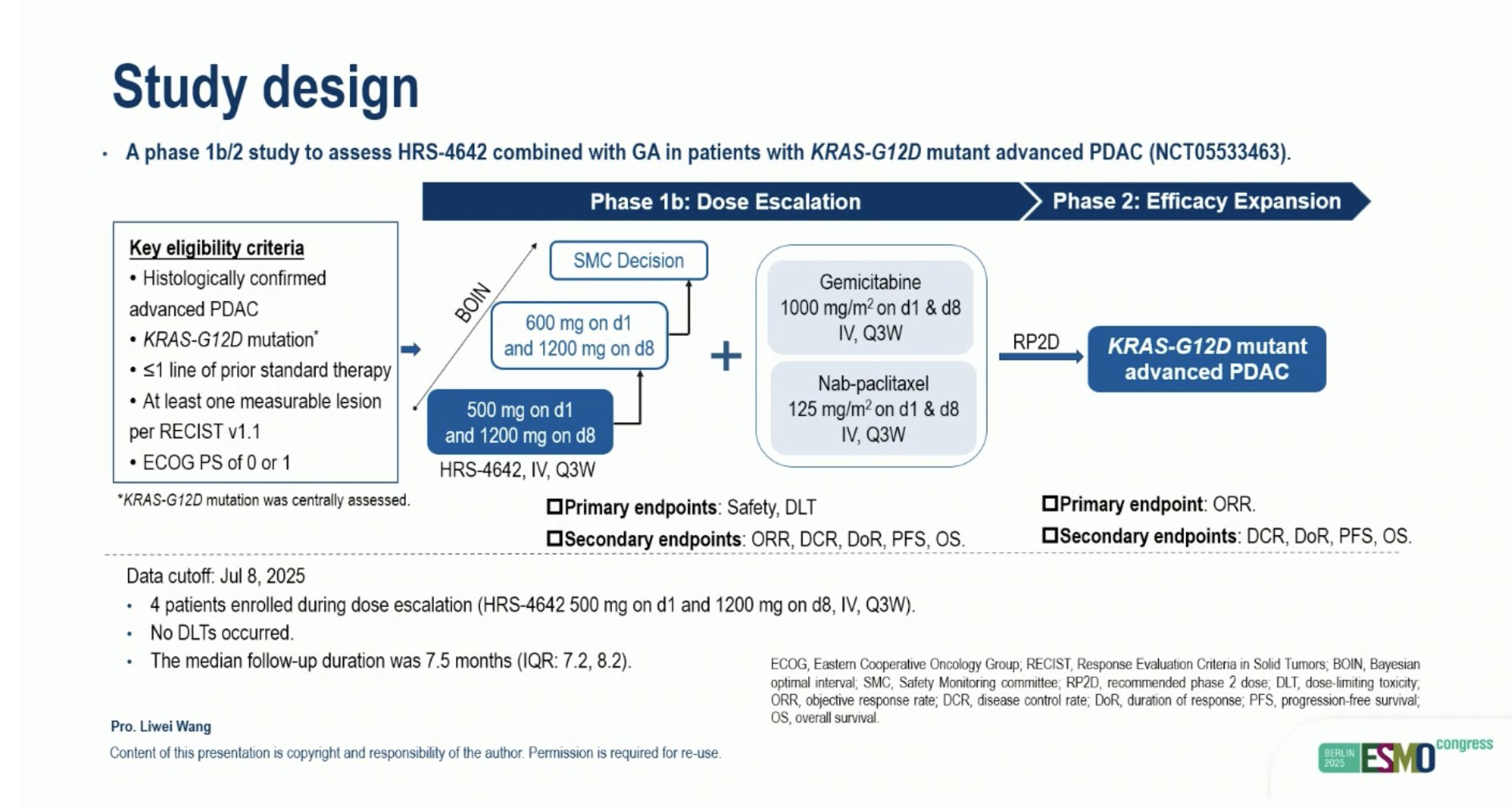
“HRS-4642 combined with gemcitabine and nab-paclitaxel in KRAS-G12D PDAC
- ORR: 63%, DCR 92%
- 6mo PFS rate: 89%
- manageable safety
KRASi are the future in 1L…”
Follow the latest ESMO 2025 news on OncoDaily.


