PATINA trial was designed to assess the efficacy and safety of incorporating palbociclib into maintenance anti-HER2 and endocrine therapy for patients with hormone-receptor–positive, HER2-positive advanced breast cancer who achieved disease control following induction chemotherapy. By targeting the CDK4/6 pathway alongside HER2 and estrogen receptor signaling, the trial sought to determine whether this approach could meaningfully extend progression-free survival in the first-line setting.
Title: Palbociclib for Hormone-Receptor–Positive, HER2-Positive Advanced Breast Cancer
Authors: Otto Metzger, M.D., Sumithra Mandrekar, Ph.D., Shom Goel, M.D., Joseph Gligorov, M.D., Elgene Lim, M.D., Eva Ciruelos, M.D., Sibylle Loibl, M.D., Travis Dockter, M.S., Xavier Gonzàlez Farré, M.D., Prudence A. Francis, M.D., Filipa Lynce, M.D., Jane Lanzillotti, M.S., Carter DuFrane, B.A., Anna Wall, B.A., Carrie Strand, B.S., Ian Krop, M.D., Ph.D., Ines Vaz-Luis, M.D., Debu Tripathy, M.D.,
Sherene Loi, M.D., Aleix Prat, M.D., Matthew Goetz, M.D., Santiago Escrivá-de-Romaní, M.D., David Porter, M.D., Jennifer Spoenlein, M.D., Daniel G. Stover, M.D., Sagar Sardesai, M.D., Pierre Heudel, M.D., Maria Koehler, M.D., Ph.D., Cynthia Huang Bartlett, M.D., Ariadna Holynskyj, M.D., Prashanth Gopalakrishna, M.D., Eric Gauthier, Pharm.D., Ph.D., Suzette Delaloge, M.D., Kathy Miller, M.D., Eric P. Winer, M.D., Luca Gianni, M.D., Ann H. Partridge, M.D., Angela DeMichele, M.D., and Lisa A. Carey, M.D.
Published in NEJM, 2026
Background
Hormone-receptor–positive, HER2-positive metastatic breast cancer represents a biologically distinct subgroup, accounting for more than half of all HER2-positive breast cancers. Standard first-line treatment consists of induction chemotherapy combined with dual HER2 blockade, most commonly trastuzumab and pertuzumab, followed by maintenance HER2-targeted therapy with endocrine treatment. Despite major advances with HER2-directed agents, resistance ultimately develops, and long-term disease control remains a clinical challenge.
Preclinical and translational studies have demonstrated significant crosstalk between HER2 signaling, estrogen-receptor pathways, and the cyclin D–CDK4/6 axis, implicating persistent CDK4/6 activity as a mechanism of resistance to both endocrine and HER2-targeted therapies. Early-phase clinical studies suggested that combining CDK4/6 inhibition with HER2-targeted and endocrine therapy is feasible and potentially effective.
The phase 3 PATINA trial was designed to determine whether adding the CDK4/6 inhibitor palbociclib to standard maintenance therapy could extend disease control in patients with hormone-receptor–positive, HER2-positive advanced breast cancer who had not progressed after induction chemotherapy.
Methods
PATINA was a randomized, open-label, phase 3 trial enrolling adults with histologically confirmed hormone-receptor–positive and HER2-positive advanced or metastatic breast cancer. Eligible patients had completed four to eight cycles of trastuzumab-based induction chemotherapy without evidence of disease progression and were enrolled within 12 weeks of completing induction therapy.
Importantly, the PATINA trial permitted the inclusion of patients with asymptomatic central nervous system (CNS) metastases at baseline, reflecting contemporary clinical practice and enhancing the external validity of the study population.
Hormone-receptor positivity was defined as ≥1% nuclear staining by immunohistochemistry, and HER2 positivity was defined as an immunohistochemistry score of 3+ or gene amplification by in situ hybridization, assessed locally. Patients with asymptomatic central nervous system metastases were eligible. The primary endpoint was investigator-assessed progression-free survival. Secondary endpoints included confirmed objective response, clinical benefit, overall survival, and safety.
Study Design
Between June 2017 and July 2021, 518 patients were enrolled across 123 sites in eight countries and randomized in a 1:1 ratio to receive maintenance anti-HER2 and endocrine therapy with palbociclib or without palbociclib. Randomization was stratified by response to:
- induction therapy (complete or partial response vs. stable disease)
- prior neoadjuvant or adjuvant anti-HER2 therapy,
- type of endocrine therapy
- use of single versus dual HER2 blockade.
Induction therapy consisted primarily of trastuzumab and pertuzumab plus a taxane, although trastuzumab alone was permitted in up to 20% of patients. Maintenance endocrine therapy included either an aromatase inhibitor or fulvestrant, with ovarian suppression required for premenopausal patients. Palbociclib was administered orally at a dose of 125 mg daily for 21 days of a 28-day cycle, with dose reductions permitted according to protocol. Tumor assessments were performed every 12 weeks using RECIST version 1.1. The final progression-free survival analysis was conducted after 262 progression or death events, with a median follow-up of 53.5 months.
Results
A total of 261 patients were assigned to the palbociclib group and 257 to the standard-therapy group. Baseline characteristics were well balanced. The median age was 53.4 years, 99.4% of patients were female, and 61.8% were postmenopausal. De novo metastatic disease was present in 54.4% of patients. At the end of induction therapy, 70.1% of patients had achieved a complete or partial response, and 29.3% had stable disease. Dual HER2 blockade was used in 94.0% of patients, and 90.7% received an aromatase inhibitor as endocrine therapy.
At a median follow-up of 53.5 months, median progression-free survival was significantly longer in the palbociclib group compared with the standard-therapy group: 44.3 months (95% CI, 32.4–56.8) versus 29.1 months (95% CI, 23.3–38.6), corresponding to a hazard ratio for disease progression or death of 0.75 (95% CI, 0.59–0.96; two-sided P=0.02). Estimated progression-free survival rates at 12, 24, and 48 months were 84.9%, 65.2%, and 46.5%, respectively, in the palbociclib group, compared with 73.2%, 55.3%, and 38.3% in the standard-therapy group.
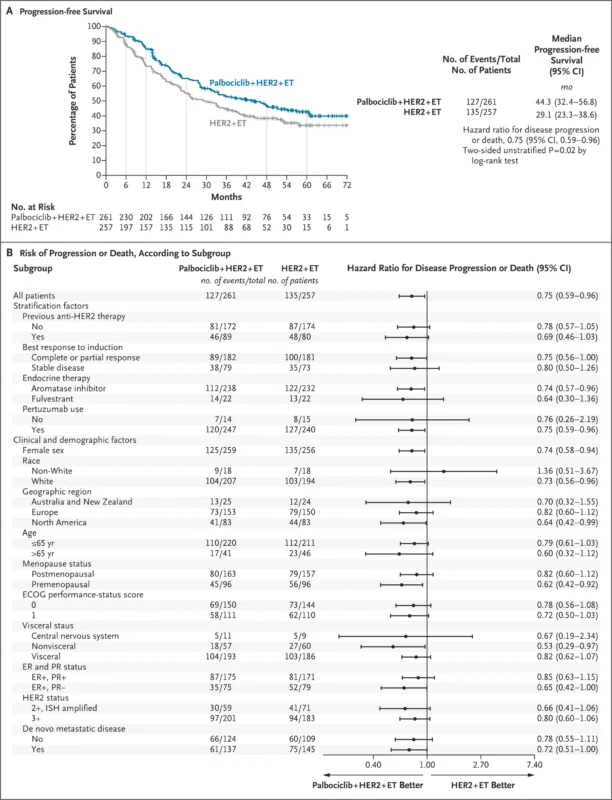
Confirmed objective responses from randomization occurred in 32.9% of patients receiving palbociclib versus 24.8% in the standard-therapy group. The median duration of confirmed response was 44.9 months with palbociclib and 30.8 months with standard therapy. Clinical benefit was observed in 88.9% of patients in the palbociclib group compared with 80.9% in the control group. At the time of data cutoff, 123 deaths had occurred (60 in the palbociclib group and 63 in the standard-therapy group), yielding a hazard ratio for overall survival of 0.86 (95% CI, 0.61–1.23). The final overall survival analysis is planned after 247 deaths.
CNS outcomes from PATINA Trial were presented by Otto Metzger during the San Antonio Breast Cancer Symposium 2025. Among patients without CNS metastases at baseline, the addition of palbociclib to anti-HER2 and endocrine therapy was associated with a significantly lower cumulative incidence of CNS progression or death compared with standard therapy alone.

At 12 months, the cumulative risk of CNS progression or death was 4.6% (95% CI, 2.5–7.9) in the palbociclib group versus 6.9% (95% CI, 4.0–10.8) in the control group. This separation increased over time, with corresponding risks of 9.7% vs. 15.7% at 24 months and 13.0% vs. 19.2% at 36 months, favoring palbociclib (Gray’s test P=0.0378).
Overall, CNS progression occurred in 12.8% (95% CI, 8.9–17.6) of patients receiving palbociclib compared with 19.0% (95% CI, 14.3–24.4) of patients in the standard-therapy group. These findings indicate that palbociclib-based maintenance therapy was associated with a lower incidence and delayed onset of CNS involvement in patients initially free of brain metastases. Although CNS-specific endpoints were exploratory and the trial was not powered for formal CNS efficacy comparisons, the consistency and durability of the observed effect suggest that concurrent inhibition of HER2, estrogen receptor signaling, and CDK4/6 may play a role in delaying or preventing CNS disease in hormone-receptor–positive, HER2-positive metastatic breast cancer, a hypothesis that warrants prospective validation.
The addition of palbociclib to maintenance anti-HER2 and endocrine therapy resulted in a clinically meaningful and statistically significant improvement in progression-free survival, extending median disease control by more than 15 months. This benefit was achieved in a population enriched for favorable biology, as patients with progression during induction therapy were excluded.
Toxicity was increased with palbociclib, driven primarily by hematologic adverse events.
- Grade 3 adverse events occurred in 79.7% of patients in the palbociclib group compared with 30.6% in the standard-therapy group.
- grade 4 events occurred in 10.0% versus 3.6%, respectively.
- Grade 3 or 4 neutropenia was reported in 60.5% of patients receiving palbociclib, with febrile neutropenia occurring in two patients.
- Palbociclib dose reductions were required in 57.7% of patients, and treatment discontinuation due to adverse events occurred in 18.0%.
Conclusion
In this phase 3 PATINA trial published in the New England Journal of Medicine, the addition of palbociclib to maintenance anti-HER2 and endocrine therapy significantly improved progression-free survival in patients with hormone-receptor–positive, HER2-positive advanced breast cancer who had not progressed after induction chemotherapy.


