Oxaliplatin-based adjuvant chemotherapy has long been a cornerstone in treating stage III colorectal cancer, but its benefit in older adults has remained uncertain. A new nationwide Korean cohort study involving more than 8,500 patients has identified age 70 as the critical threshold for survival benefit. Patients aged 70 years or younger with stage III disease experienced significantly improved overall survival with oxaliplatin, while those older than 70 saw no measurable advantage and had higher treatment discontinuation rates. In stage II colorectal cancer, oxaliplatin showed no survival benefit at any age, underscoring the need for more tailored treatment strategies based on age, comorbidities, and functional status.
Title: Older Age Threshold for Oxaliplatin Benefit in Stage II to III Colorectal Cancer
Authors: Jun Woo Bong, MD, PhD, Hwamin Lee, PhD, Seogsong Jeong, MD, PhD, Sanghee Kang, MD, PhD
Background
Colorectal cancer remains a leading cause of cancer-related mortality despite advances in screening and therapy. Surgical resection followed by adjuvant chemotherapy is the standard for nonmetastatic disease. The role of oxaliplatin in older patients, however, remains controversial. Evidence from trials such as MOSAIC and pooled ACCENT analyses suggested limited benefit of oxaliplatin in patients aged ≥65 years, while some retrospective studies reported survival improvements even in those over 70 years. Neurotoxicity and higher discontinuation rates in older patients add complexity to treatment decisions. This nationwide Korean study aimed to define an optimal age threshold for oxaliplatin benefit in stage II–III colorectal cancer.
Methods and Study Design
Data were obtained from the Korea Health Insurance Review and Assessment Service (HIRA) National Quality Assessment (NQA) program. This retrospective cohort included patients with pathologically confirmed stage II–III colorectal cancer who underwent curative radical resection between January 2014 and December 2016 and initiated adjuvant chemotherapy within 2 months post-surgery.
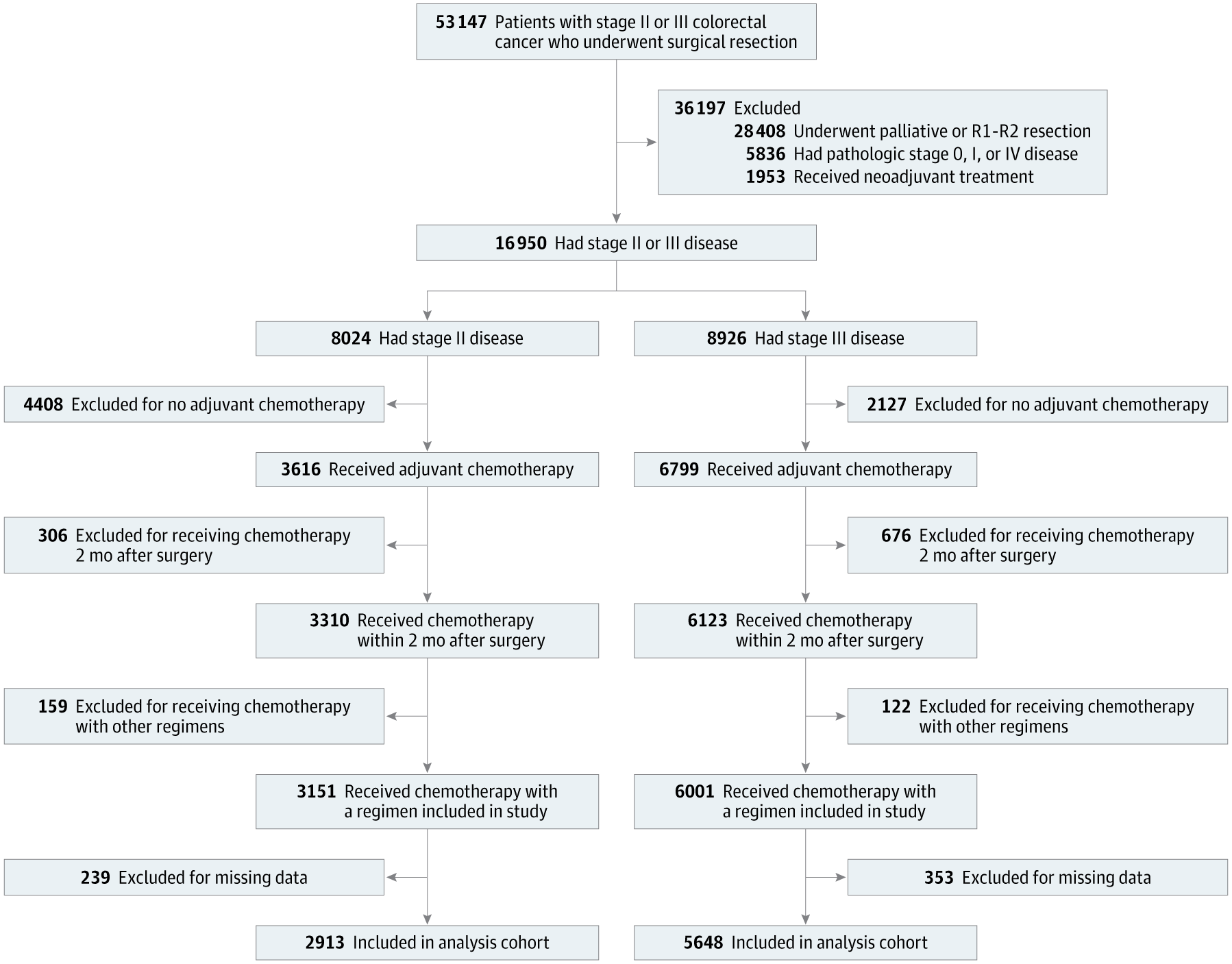
Eligible patients received
- non-oxaliplatin regimen (fluorouracil + leucovorin or capecitabine)
- oxaliplatin-based regimen (CAPOX, FOLFOX, or modified FOLFOX)
Exclusions included receipt of neoadjuvant therapy, participation in clinical trials of other chemotherapy agents, delayed initiation of adjuvant chemotherapy (>2 months post-surgery), or missing data.
The primary endpoint was overall survival (OS), assessed from surgery until death or April 30, 2024. Cox proportional hazards models were applied in crude, minimally adjusted, and fully adjusted analyses, incorporating age, sex, body mass index, comorbidity indices, tumor histology, lymph node count, regimen type, and chemotherapy discontinuation status. Age thresholds from 60 to 80 years were evaluated to determine where oxaliplatin conferred benefit. Propensity score matching (PSM, 1:1, caliper 0.1) was performed to reduce selection bias. Sensitivity analyses excluded deaths within 6 or 12 months of follow-up.
Results
The analysis of the Korean nationwide cohort provided a detailed breakdown of patient characteristics, survival outcomes, and treatment patterns. Differences in age distribution, stage, and regimen choice highlighted how clinical practice varies between younger and older patients — and how these factors influenced the benefits and risks of oxaliplatin-based chemotherapy.
Patient Population
Out of 53,147 patients screened, 8,561 met inclusion criteria (mean age 63.2 years; 40.6% female). Stage II disease accounted for 34.0% (n = 2,913) and stage III for 65.9% (n = 5,648). Oxaliplatin-based regimens were used more often in stage III than in stage II disease. Among patients ≤70 years, only 8.9% received non-oxaliplatin therapy compared with 38.6% in those >70 years.
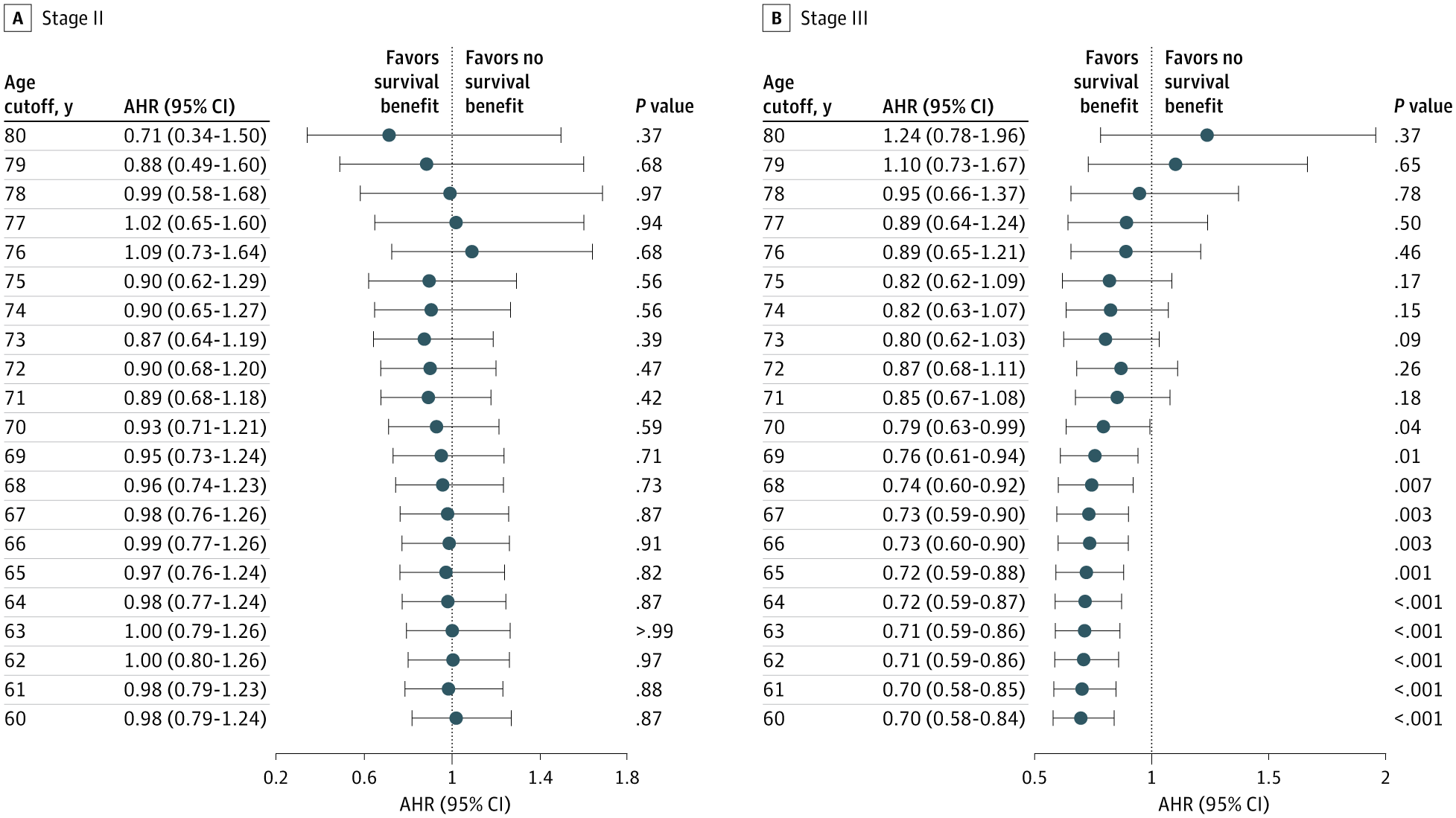
Age Threshold Analysis
- Stage II: No statistically significant overall survival (OS) benefit at any age threshold from 60–80 years (AHR range: 0.71–1.09).
- Stage III: Optimal cutoff identified at 70 years.
- ≤70 years: Significant OS improvement with oxaliplatin (AHR = 0.79, P = .04; fully adjusted HR before PSM = 0.59, 95% CI 0.46–0.77, P < .001).
- >70 years: No survival benefit (fully adjusted HR = 0.85, 95% CI 0.67–1.07, P = .18).
Survival Outcomes
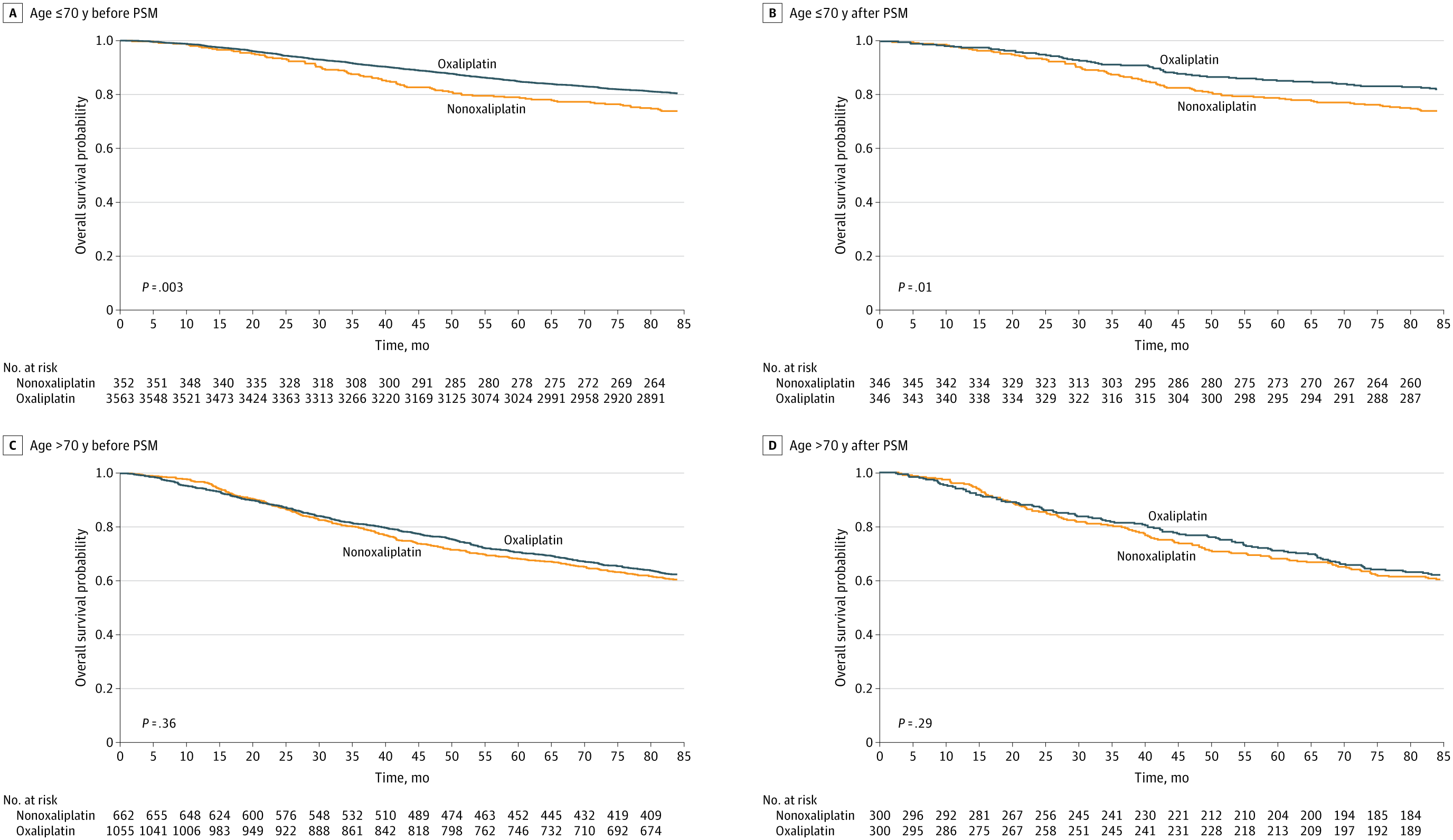
Stage III, ≤70 years:
- Before PSM: 5-year OS = 84.8% (oxaliplatin) vs 78.1% (non-oxaliplatin), P = .003.
- After PSM: 85.0% vs 78.9%, P = .01.
Stage III, >70 years:
- Before PSM: 70.6% vs 68.3%, P = .36.
- After PSM: 71.0% vs 68.0%, P = .29.
Chemotherapy Discontinuation
- Increased with age: 37.4% in >70 years vs 23.9% in ≤70 years (P < .001).
- In >70 years, oxaliplatin use was linked to higher discontinuation (39.9% vs 33.4%, P = .008; AOR = 1.55, 95% CI 1.19–2.03, P = .001).
Discontinuation was associated with worse OS:
- ≤70 years: AHR = 1.79, 95% CI 1.56–2.06, P < .001.
- 70 years: AHR = 1.54, 95% CI 1.33–1.79, P < .001.
- In ≤70 years, stopping oxaliplatin but continuing fluoropyrimidine worsened OS if dose intensity fell below 80% (AHR = 1.72, 95% CI 1.12–2.62, P = .01).
Key Findings
- Stage III, ≤70 years: Clear OS benefit from oxaliplatin.
- Stage III, >70 years: No OS benefit; higher discontinuation.
- Stage II: No OS benefit at any age.
- Toxicity Burden: Older adults faced higher discontinuation rates and likely more neurotoxicity.
- Treatment Duration Evidence: SCOT and ACHIEVE-2 trials suggest 3-month CAPOX regimens may reduce neurotoxicity without compromising survival in select patients.
Key Takeaway Messages
- Age 70 is a practical threshold for predicting OS benefit from oxaliplatin in stage III colorectal cancer.
- For patients over 70, the risks, especially treatment discontinuation and toxicity, may outweigh survival advantages.
- In stage II disease, the incremental benefit of oxaliplatin is negligible regardless of age, reinforcing the role of risk stratification.
- Shorter-duration or lower-intensity regimens may optimize tolerability in older or frail patients without sacrificing efficacy.
- Treatment decisions should be guided by biologic age, comorbidity burden, and functional status rather than chronological age alone.
Conclusion
In this large, nationwide Korean cohort, oxaliplatin-based adjuvant chemotherapy significantly improved overall survival in stage III colorectal cancer patients aged ≤70 years but conferred no benefit in those older than 70 years or in any patients with stage II disease. Higher discontinuation rates in older adults highlight the importance of individualized treatment planning, incorporating geriatric assessments and balancing efficacy with tolerability. These findings support refining adjuvant chemotherapy guidelines to better align with patient age, comorbidities, and functional capacity, rather than applying a uniform treatment approach across all age groups.
You can read the full article here.
Written by Sona Karamyan.