OPBC-07/microNAC addresses one of the most debated questions in contemporary breast cancer surgery: how to optimally manage the axilla after neoadjuvant chemotherapy (NAC), particularly in patients with residual low-volume nodal disease. While randomized trials in the upfront surgery setting have established that axillary lymph node dissection (ALND) can be safely omitted in selected patients with limited sentinel-node involvement, evidence in the post-neoadjuvant setting remains far less robust. Patients with residual micrometastases (ypN1mi) after NAC represent a clinically challenging group, as prior studies have demonstrated a substantial rate of additional nodal disease at completion ALND, raising concerns regarding regional disease control.
Despite this uncertainty, real-world practice has increasingly shifted toward ALND omission combined with regional nodal irradiation. The OPBC-07/microNAC study was designed to address this evidence gap by evaluating oncological outcomes with and without ALND in a large international cohort of patients with ypN1mi disease after NAC, with particular attention to the role of tumor biology in shaping axillary recurrence risk.
Background
Axillary lymph node dissection (ALND) has historically been recommended for patients who have residual nodal disease after neoadjuvant chemotherapy (NAC), because residual disease may reflect a higher risk of regional recurrence. At the same time, ALND carries meaningful morbidity, particularly lymphoedema, shoulder dysfunction, and sensory symptoms. In upfront surgery, randomized trials have shown that ALND can be safely avoided in selected patients with limited sentinel-node involvement, including micrometastases, when modern radiotherapy and systemic therapy are used.
However, the post-neoadjuvant setting is different: studies have shown that when sentinel nodes remain positive after NAC, the likelihood of additional involved nodes at completion ALND can be substantial, reported in the range of roughly one-quarter to more than half for ypN1mi in prior series. Because real-world practice has increasingly moved toward omitting ALND in favor of regional nodal irradiation—despite limited outcome data—OPBC-07/microNAC was designed to quantify recurrence outcomes in a large international cohort of patients with residual sentinel-node micrometastases (ypN1mi) after NAC, treated with or without completion ALND.
Methods
This was a retrospective cohort study drawing from institutional databases at 84 cancer centers in 30 countries, including both high-volume academic units and smaller breast services to improve generalizability. Eligible patients were ≥18 years with clinical T1–4, N0–3 breast cancer at diagnosis, treated with neoadjuvant chemotherapy followed by surgery between Jan 1, 2013 and May 31, 2023. All patients had residual micrometastases in axillary nodes (defined as metastasis >0.2 mm or >200 cells, and ≤2.0 mm) identified after axillary staging with sentinel lymph node biopsy (SLNB), targeted axillary dissection (TAD), or the MARI procedure.
For patients who initially presented with palpable nodal disease, downstaging to clinical N0 after NAC was required. Patients with inflammatory breast cancer, stage IV disease, those who underwent ALND as the primary procedure, and those who received neoadjuvant endocrine therapy were excluded. Patients with isolated tumor cells only or macrometastatic disease in sentinel/target nodes were not eligible.
The primary endpoint was the rate of any axillary recurrence (isolated or combined with local or distant recurrence) by axillary surgery approach (ALND vs no ALND). Secondary endpoints included locoregional recurrence, any invasive recurrence (locoregional or distant), and the proportion of additional positive nodes at completion ALND. Because the median follow-up was 3.1 years, the investigators reported 3-year cumulative incidence estimates and exploratory 5-year estimates. Analyses used competing-risk methods (Gray’s test for subgroup comparisons).
Study Design
OPBC-07/microNAC was an international, retrospective, multicenter cohort study that analyzed data from institutional databases of 84 cancer centers across 30 countries. The study included patients with breast cancer who had residual sentinel-node micrometastases (ypN1mi) after neoadjuvant chemotherapy (NAC) and were treated with sentinel lymph node biopsy, targeted axillary dissection, or the MARI procedure, with or without completion axillary lymph node dissection (ALND).
Surgeries were performed between 2013 and 2023, and the study was registered on ClinicalTrials.gov (NCT06529302). The median follow-up was 3.1 years (IQR 1.8–5.2), allowing assessment of early and intermediate oncological outcomes in a contemporary treatment setting characterized by a high utilization of regional nodal radiotherapy, reflecting current multidisciplinary breast cancer practice.
Results
A total of 1,585 women with ypN1mi disease were analyzed. 804 (50.7%) underwent completion ALND, and 781 (49.3%) did not. The cohort’s median age was 48 years (IQR 41–58). Regarding disease burden at presentation, 58.4% had cT2 tumors and 66.5% were clinically node-positive at diagnosis. Importantly, 79.9% (1,267/1,585) received regional nodal irradiation, a key contextual factor when interpreting recurrence risk.
Tumor subtypes were distributed as follows: 51.0% hormone receptor–positive/HER2-negative (808/1,585), 31.1% HER2-positive (493/1,585, combining HR+ and HR– HER2+), and 17.9% triple-negative (284/1,585). In the ALND group, patients were more likely to have presented with clinically node-positive disease (72.4% vs 60.4%), and micrometastases were more commonly detected intraoperatively on frozen section (62.4% vs 17.3%), consistent with real-world selection of higher-risk or higher-certainty cases for completion ALND.
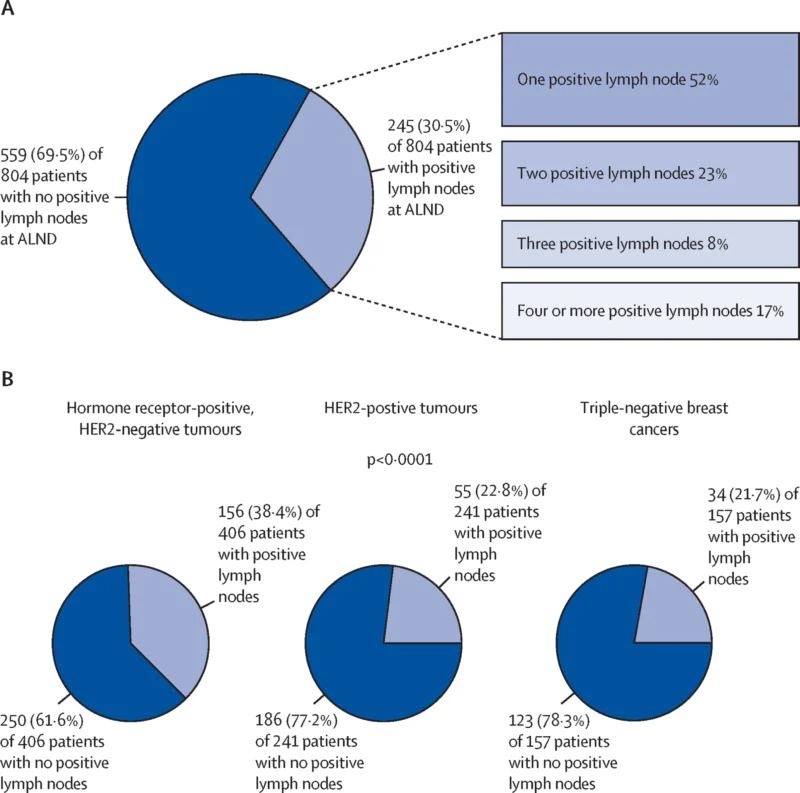
Among the 804 patients who had ALND, additional positive nodes were found in 245 (30.5%). The composition of these additional findings included isolated tumor cells in 20 (8.2%), micrometastases in 123 (50.2%), and macrometastases in 102 (41.6%)—showing that even when residual disease is “low-volume” in sentinel/target nodes, a substantial fraction has more extensive residual nodal burden at completion ALND. There was also a statistically significant (though modest) correlation between the number of sentinel nodes with micrometastases and the number of additional positive nodes at ALND (Pearson r 0.16; p<0.0001).
Despite this residual burden, axillary recurrence outcomes were low overall. Across the entire cohort, there were 34 any axillary recurrences (2.1%) and 7 isolated axillary recurrences (0.4%) during follow-up. The 3-year cumulative incidence of any axillary recurrence was 2.0% (95% CI 1.3–2.9), and isolated axillary recurrence was 0.3% (0.1–0.7). When comparing surgical strategies, 3-year any axillary recurrence was 1.7% (95% CI 0.9–2.9) with ALND versus 2.3% (1.4–3.7) without ALND, with no significant difference. Similarly, the 3-year locoregional recurrence rate was 4.1% overall, and any invasive recurrence was 14% (12–16), with no meaningful differences between ALND and no-ALND groups for these endpoints in the full cohort.
A key biological signal emerged in triple-negative breast cancer (TNBC). In TNBC patients, omission of ALND was associated with significantly higher any axillary recurrence: 8.7% (95% CI 4.4–15.0) without ALND versus 2.4% (0.7–6.5) with ALND (p=0.018). Notably, any invasive recurrence in TNBC was high but similar between strategies (35% vs 32%, not statistically different), indicating that ALND primarily affected regional control rather than overall invasive recurrence during the available follow-up.
On multivariable analysis, omission of ALND was not independently associated with increased risk of axillary recurrence (HR 0.86; 95% CI 0.37–2.00) or invasive recurrence (HR 1.05; 0.78–1.41). In contrast, TNBC biology and omission of regional nodal irradiation were independently associated with higher axillary recurrence risk. Specifically, TNBC had an increased hazard for any axillary recurrence (HR 3.83; 1.72–8.52) and for any invasive recurrence (HR 3.17; 2.30–4.35). Not receiving nodal irradiation increased risk for any axillary recurrence (HR 2.62; 1.19–5.73) and invasive recurrence (HR 1.61; 1.20–2.15). These patterns were consistent across propensity-matched and sensitivity analyses, supporting the robustness of the main conclusion.
Exploratory 5-year estimates also remained low: any axillary recurrence 2.7% (95% CI 1.8–3.8) and isolated axillary recurrence 0.49% (0.19–1.1), with no meaningful differences by ALND use overall.
Key findings
- ALND omission was common: nearly half of ypN1mi patients (49.3%) did not undergo completion ALND.
- Regional nodal irradiation was widely used (79.9%), shaping modern outcomes.
- Overall axillary recurrence was low: 3-year any axillary recurrence 2.0%; isolated axillary recurrence 0.3%.
- No overall recurrence advantage for ALND: 3-year axillary recurrence 1.7% vs 2.3% (ALND vs no ALND), with similar locoregional and invasive recurrence.
- Residual burden at ALND was substantial: additional positive nodes in 30.5% of ALND patients, including 41.6% macrometastases among those with additional disease.
- Tumor biology mattered: TNBC patients had higher axillary recurrence without ALND (8.7% vs 2.4%).
- Independent risk factors for axillary recurrence were TNBC and omission of nodal radiotherapy, not omission of ALND in the overall population.
Conclusion
OPBC-07/microNAC provides large-scale international evidence that, for most patients with residual sentinel-node micrometastases (ypN1mi) after neoadjuvant chemotherapy who receive regional nodal irradiation, omission of completion ALND is associated with very low axillary recurrence and does not worsen short-term invasive outcomes.
However, the findings also highlight a biologically defined exception: in triple-negative breast cancer, omission of ALND was linked to a significantly higher rate of axillary recurrence, suggesting that surgical de-escalation should be approached cautiously in this subgroup. Overall, the study supports modern, subtype-aware axillary de-escalation after NAC, while reinforcing the importance of nodal radiotherapy and tumor biology in guiding safe treatment decisions.


