The NOBEL trial explored the integration of the PD-1 inhibitor nivolumab into definitive cisplatin–fluorouracil chemoradiotherapy for patients with operable and inoperable oesophageal squamous cell carcinoma. Designed to address long-standing questions around safety, treatment deliverability, and biological activity, the study investigated whether immune checkpoint blockade could be combined with curative-intent chemoradiotherapy without compromising tolerability, while laying the groundwork for biomarker-guided patient selection in this disease setting.
Title: Nivolumab with definitive chemoradiotherapy for oesophageal squamous cell carcinoma (NOBEL): a multicentre, single-arm, phase 2 feasibility trial
Authors: Motoo Nomura, Katsuyuki Sakanaka, Juko Shimizu, Shinya Ohashi, Chikatoshi Katada, Akinori Watanabe, Yusuke Amanuma, Keiko Minashi, Ken Kato, Takashi Kojima, Kengo Nagashima, Ihhwa Kim, Harue Tada, Akiyoshi Nakakura, Manabu Muto
Published in Lancet Oncology, January 2026
Background
Oesophageal cancer is the seventh most common cancer worldwide.Oesophageal squamous cell carcinoma (OSCC) is the predominant histological type globally, particularly in Asian countries. Definitive chemoradiotherapy (dCRT) using cisplatin and 5-fluorouracil (5-FU) with approximately 50 Gy of radiation is a curative-intent option for patients who are inoperable or who decline surgery, but long-term control remains suboptimal. Immune checkpoint inhibitors (ICIs), particularly PD-1 inhibitors such as nivolumab, have demonstrated survival benefit in recurrent or metastatic OSCC. Preclinical and clinical data from other tumour types suggest that combining ICIs with chemoradiotherapy may enhance antitumour immunity.
However, safety—especially pneumonitis—remains a key concern when integrating immunotherapy with thoracic radiation. The NOBEL trial was designed to assess the feasibility, safety, and preliminary efficacy of adding nivolumab to definitive cisplatin/5-FU chemoradiotherapy in both operable and inoperable OSCC.
Methods
NOBEL was a multicentre, open-label, single-arm phase 2 feasibility study conducted at five Japanese institutions. Eligible patients were aged 20–75 years with histologically confirmed thoracic OSCC, ECOG performance status 0–1, and adequate organ function. Patients were enrolled if they were suitable for definitive CRT either because they had operable disease but refused surgery or had inoperable disease (including T4 tumours or extensive nodal involvement). Patients with oesophago-respiratory fistula, major bleeding, or arterial invasion were excluded.
Treatment consisted of concurrent chemoradiotherapy with cisplatin (75 mg/m² day 1) and 5-FU (1000 mg/m²/day days 1–4) combined with nivolumab (240 mg flat dose on days 1 and 15) every 28 days during radiotherapy. Radiotherapy was delivered to a total dose of 50.4 Gy in 28 fractions. After definitive treatment, patients received two additional cycles of immunochemotherapy followed by nivolumab maintenance for up to one year.
Toxicities were graded using CTCAE v5.0. Because immune-related and radiation-related pneumonitis are difficult to distinguish, all pneumonitis events were classified as treatment-related and centrally reviewed. Tumour response was assessed using RECIST 1.1 and standardized endoscopic criteria, with complete response (CR) requiring confirmation on repeat evaluation at least four weeks apart.
Exploratory biomarker analysis evaluated the expression of 51 immune-related genes from pretreatment biopsy specimens to identify immune activation subtypes.
Study Design
The primary endpoint was safety, defined as ≤10% incidence of grade ≥4 non-haematologic toxicity and ≤15% incidence of grade ≥3 pneumonitis. Secondary endpoints included complete response rate, progression-free survival (PFS), overall survival (OS), and major PFS. The trial was registered as jRCT1091220408 and was terminated early due to slow accrual.
Results
Between January 2019 and September 2021, 42 patients were enrolled and included in the safety analysis set; 41 patients were evaluable for efficacy. Of these, 25 had operable disease and 16 had inoperable disease. Median follow-up was 28.7 months.
Treatment delivery was feasible, with median relative dose intensities of 92% for nivolumab, cisplatin, and 5-FU. Forty patients completed definitive immune-chemoradiotherapy, and 36 received nivolumab maintenance; 56% completed the full one-year maintenance period.
The primary safety endpoint was met. Grade 3 pneumonitis occurred in 2 patients (5%), well below the prespecified 15% threshold, and no treatment-related deaths were observed. Pneumonitis of any grade occurred in 79% of patients, reflecting broad capture of inflammatory lung events during and after CRT. The most frequent grade ≥3 toxicities were lymphopenia (86%), decreased white blood cell count (43%), and neutropenia (38%). Grade ≥3 oesophagitis occurred in 19% of patients.
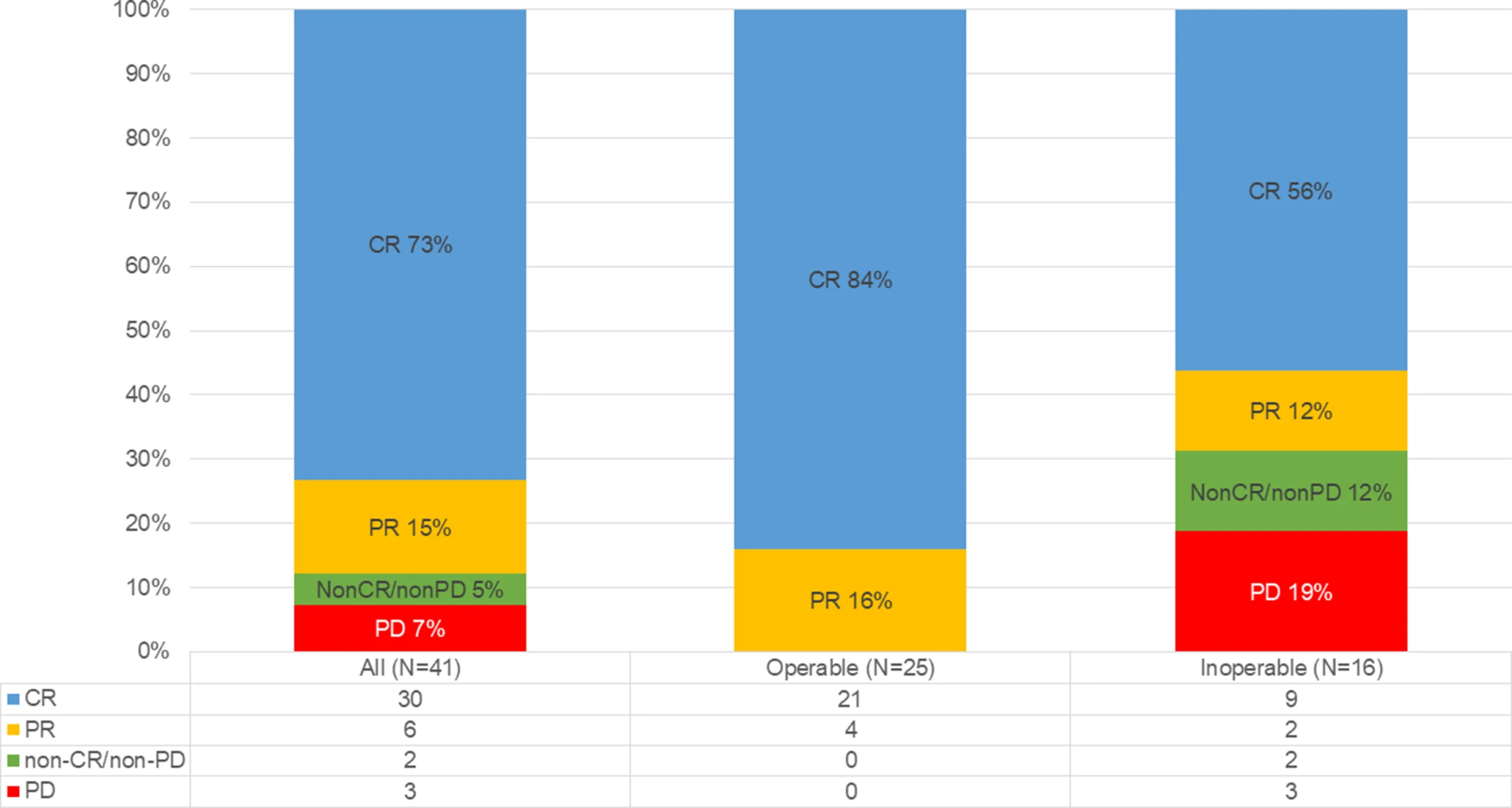
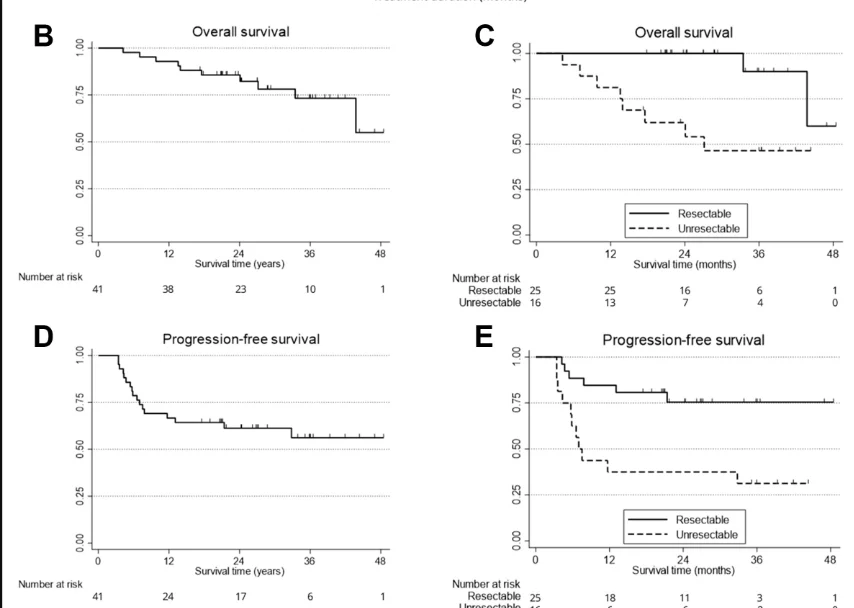
Efficacy outcomes were encouraging. The best overall complete response rate was 73% (30/41; 95% CI 58–84), with a median time to confirmed CR of 130 days. CR rates differed by resectability: 84% in operable patients and 56% in inoperable patients. One-year OS was 92.7% (95% CI 79.0–97.6), including 100% in operable patients and 81.3% in inoperable patients. One-year PFS was 65.4% (95% CI 48.6–77.9).
Nine patients received salvage therapy after recurrence or non-response, including surgery, endoscopic resection, or systemic therapy. No treatment-related deaths occurred following salvage procedures.
Exploratory biomarker analysis identified two immune gene-expression subtypes: immune high-active (n=5) and immune moderate-active (n=37). All evaluable patients in the immune high-active group achieved complete response, compared with a 70% CR rate in the moderate-active group, although numbers were small and findings were hypothesis-generating.
Key findings
- Nivolumab can be safely combined with definitive cisplatin/5-fluorouracil chemoradiotherapy in oesophageal squamous cell carcinoma.
- Severe pneumonitis was uncommon, and no treatment-related deaths were reported.
- The regimen achieved high complete response rates with favourable one-year survival outcomes in both operable and inoperable disease.
- Immune gene-expression profiling showed potential to identify patients most likely to achieve deep and durable responses.
Conclusion
The NOBEL phase 2 feasibility trial shows that nivolumab combined with definitive chemoradiotherapy is deliverable and safe in OSCC, with promising efficacy signals and manageable toxicity. Although limited by its single-arm design, small sample size, and early termination, the study provides a strong rationale for randomized trials to confirm benefit and to refine biomarker-driven strategies for immune-chemoradiotherapy in oesophageal squamous cell carcinoma.
You can read the full article here.


