KEYVIBE-010 trial evaluated whether adding TIGIT inhibition with vibostolimab to pembrolizumab could improve recurrence-free survival compared with pembrolizumab alone in resected stage IIB–IV melanoma. Among 1402 randomized patients, the first interim futility analysis showed no benefit (HR 1.25; 95% CI 0.9–1.8) and higher immune-related toxicity with the combination, supporting pembrolizumab monotherapy as the adjuvant standard.
Background
Adjuvant anti–PD-1 therapy has become a standard approach for patients with completely resected, high-risk cutaneous melanoma because it reduces recurrence risk across stage IIB–III disease. Even with PD-1 inhibitors, a meaningful proportion of patients still relapse, so intensified checkpoint strategies have been pursued. TIGIT is an inhibitory receptor expressed on T cells, regulatory T cells, and NK cells; mechanistically, dual TIGIT and PD-1 blockade is hypothesized to reinvigorate anti-tumor immunity more effectively than PD-1 blockade alone.
Vibostolimab is an anti-TIGIT IgG1 antibody designed to block TIGIT–ligand interactions and engage Fcγ receptors on myeloid cells, providing a rationale for pairing with pembrolizumab in earlier-stage melanoma. The phase 3 KEYVIBE-010 study tested whether a fixed-dose coformulation of vibostolimab plus pembrolizumab could improve recurrence outcomes compared with pembrolizumab alone in the adjuvant setting.
Methods
KEYVIBE-010 enrolled patients aged ≥12 years with surgically resected stage IIB–IV cutaneous melanoma (AJCC 8th edition) and no evidence of metastatic disease after resection. Key exclusions included prior systemic therapy for melanoma, CNS metastases/carcinomatous meningitis, and non-cutaneous melanoma subtypes (ocular, mucosal, conjunctival). Performance status requirements were age-appropriate (ECOG 0–1 for adults). Imaging surveillance was intensive early (every 12 weeks for 2 years), then spaced out through year 5. The primary endpoint was recurrence-free survival (RFS) in the intention-to-treat population; safety was assessed in patients who received at least one dose. A protocol-prespecified first interim analysis served as an event-driven nonbinding futility check.
Study Design
This was a randomised, double-blind, phase 3 trial across 205 global sites, with 1:1 allocation to:
- Vibostolimab 200 mg coformulated with pembrolizumab 200 mg IV every 3 weeks,or
- Pembrolizumab 200 mg IV every 3 weeks (weight-based dosing for participants <18 years, capped at 200 mg)
for up to 17 cycles (or until recurrence/progression, unacceptable toxicity, etc.). Randomisation was stratified by risk-based stage (IIB–IIIB vs IIIC–IV) and geographic region (Asia vs rest of world). Importantly, dose reductions were not permitted; treatment could be interrupted/withdrawn to manage immune-related toxicity.
The first interim analysis was planned around 111 RFS events, with a futility boundary defined as an observed hazard ratio (HR) >0.95 for RFS; crossing this boundary would support stopping for lack of benefit.
Results
Between Jan 19, 2023 and March 6, 2024, 1402 participants were randomised: 701 to vibostolimab–pembrolizumab and 701 to pembrolizumab alone. Median age was 61.0 years (IQR 51.0–70.0). Although eligibility allowed ≥12 years, no participants <18 years were enrolled (youngest age reported was 21). Sex distribution was balanced (about 59% male, 41% female). Stage distribution reflected a broad high-risk population: ~60% had stage IIB–IIIB, and ~39% had stage IIIC–IV disease.
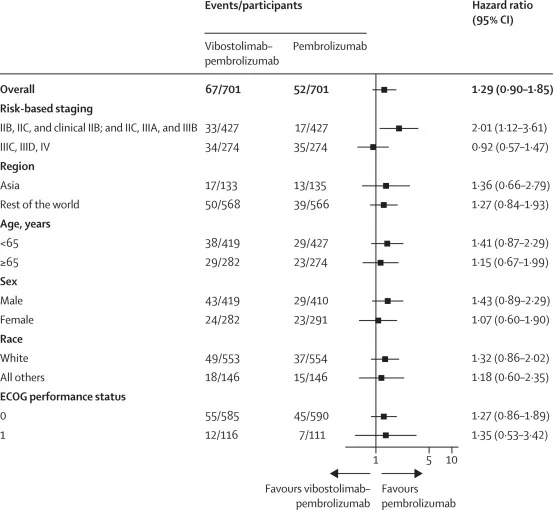
At data cutoff (March 6, 2024), the median follow-up was 4.2 months (IQR 1.9–6.7), making this an intentionally early look designed to detect futility rather than long-term separation. At that time, 119 RFS events (8%) had occurred: 67/701 (10%) in the vibostolimab–pembrolizumab arm versus 52/701 (7%) in the pembrolizumab arm. The median RFS was not reached in either group. The estimated HR for RFS was 1.25 (95% CI 0.9–1.8), numerically favoring pembrolizumab alone rather than the combination. The 6-month RFS estimates were 80% (95% CI 75–85) with vibostolimab–pembrolizumab and 85% (81–89) with pembrolizumab. Subgroup analyses did not show a consistent RFS advantage for the combination across prespecified strata.
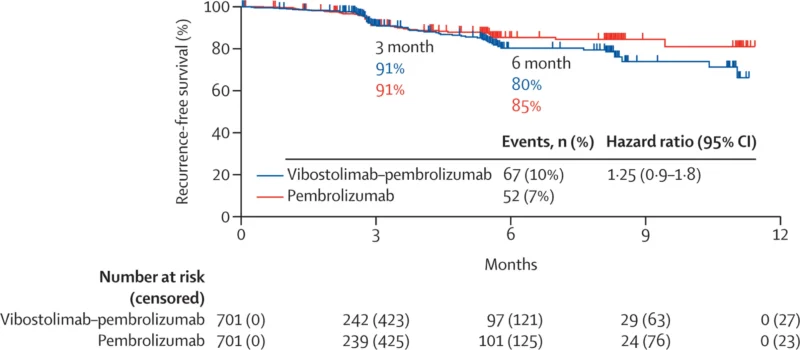
Because the interim analysis met the prespecified futility criterion, the external data monitoring committee recommended discontinuation of the study regimen. The trial was unmasked and patients receiving the coformulation were offered the option to switch to pembrolizumab monotherapy. This outcome aligns with the sponsor’s earlier public update indicating the program would be halted based on futility in RFS.
Toxicity burden was higher with dual checkpoint therapy, without offsetting efficacy:
- All-cause adverse events: 82% vs 80% (vibostolimab–pembrolizumab vs pembrolizumab).
- Grade ≥3 all-cause adverse events: 22% vs 11%.
- Treatment-related adverse events (TRAEs): 74% vs 66%.
- Grade 3–5 TRAEs: 16% vs 7%.
- Treatment-related serious adverse events: 11% vs 4%.
- TRAEs leading to discontinuation: 12% vs 6%.
The most common treatment-related events with the coformulation included pruritus (24%), rash (23%), and fatigue (16%). Grade ≥3 TRAEs more frequently reflected immune toxicities (eg, adrenal insufficiency 2%, hepatitis 2%, and severe rash/pruritus around ~1%). Importantly, treatment-related deaths occurred in 2 (<1%) patients on vibostolimab–pembrolizumab (reported causes included myasthenia gravis and myocarditis) and 1 (<1%) patient on pembrolizumab (reported as myositis). Immune-mediated adverse events/infusion reactions were also higher with the combination (30% vs 24%), and grade ≥3 immune-mediated events were 12% vs 4%, consistent with additive immune toxicity from dual checkpoint blockade.
Key findings
KEYVIBE-010 did not demonstrate clinical benefit for adding anti-TIGIT therapy to pembrolizumab in adjuvant resected stage IIB–IV melanoma, based on an early event-driven interim analysis. RFS was not improved (HR 1.25, 95% CI 0.9–1.8), and early 6-month RFS estimates numerically favored pembrolizumab alone (85% vs 80%). Meanwhile, the combination produced substantially higher immune-related toxicity and higher rates of serious and grade ≥3 adverse events.
Conclusion
In the randomized, double-blind phase 3 KEYVIBE-010 trial, vibostolimab coformulated with pembrolizumab failed to improve recurrence-free survival versus pembrolizumab alone as adjuvant therapy for resected stage IIB–IV cutaneous melanoma, triggering protocol-defined futility at the first interim analysis. Despite strong mechanistic rationale for dual TIGIT/PD-1 blockade and encouraging signals in earlier studies, the combination produced more toxicity—especially immune-mediated events and serious treatment-related adverse events—without evidence of added efficacy.


