KEYNOTE-905/EV-303 was a phase 3 trial evaluating perioperative enfortumab vedotin plus pembrolizumab in patients with muscle-invasive bladder cancer who were ineligible for or declined cisplatin-based chemotherapy. The study demonstrated that integrating this antibody–drug conjugate and PD-1 inhibitor before and after radical cystectomy significantly improved clinical outcomes compared with surgery alone, establishing a new perioperative treatment option for this high-risk population.
Title: Perioperative Enfortumab Vedotin and Pembrolizumab in Bladder Cancer
Authors: Christof Vulsteke, M.D., Ph.D., Nabil Adra, M.D., Pongwut Danchaivijitr, M.D., Maksym Sabadash, M.D., Ph.D., Alejo Rodriguez-Vida, M.D., Ph.D., Zhentao Zhang, M.D., Ph.D., Vagif Atduev, M.D., Y. Emre Göger, M.D., Steffen Rausch, M.D., Seok-Ho Kang, M.D., Ph.D., Yohann Loriot, M.D., Ph.D., Jens Bedke, M.D., Matthew D. Galsky, M.D., Peter H. O’Donnell, M.D., Gunhild von Amsberg, M.D., Nimira Alimohamed, M.D., Grzegorz Sulimka, M.D., Shilpa Gupta, M.D., Viktor Paramonov, M.D., Keita Nakane, M.D., Ph.D., Michael Mihm, Ph.D., Changting Meng, M.D., Caizhi David Huang, Ph.D., Chethan Ramamurthy, M.D., Blanca Homet Moreno, M.D., Ph.D., Anders Ullén, M.D., Ph.D.
Background
Muscle-invasive bladder cancer (MIBC) is an aggressive disease in which radical cystectomy with pelvic lymph-node dissection remains the standard curative-intent approach. Neoadjuvant cisplatin-based chemotherapy improves survival and is the preferred perioperative strategy; however, nearly half of patients with MIBC are ineligible for cisplatin due to impaired renal function, poor performance status, heart failure, or other comorbidities as defined by the Galsky criteria.
These patients often proceed directly to surgery and have inferior outcomes, with reported median event-free survival (EFS) around 12 months and approximately one third remaining event-free at 3 years. Enfortumab vedotin, a nectin-4–directed antibody–drug conjugate, combined with pembrolizumab, an anti–PD-1 antibody, has demonstrated robust activity in advanced or metastatic urothelial carcinoma irrespective of cisplatin eligibility.
The phase 3 KEYNOTE-905/EV-303 trial evaluated whether this combination, administered perioperatively (neoadjuvant and adjuvant), could improve outcomes compared with surgery alone in patients with MIBC who were ineligible for or declined cisplatin-based chemotherapy.
Methods
This was a randomized, open-label, multicenter, phase 3 trial conducted across 242 sites in 27 countries. Eligible patients were adults with clinically nonmetastatic MIBC (T2–T4aN0M0 or T1–T4aN1M0), predominantly urothelial histology (≥50%), ECOG performance status 0–2, and either ineligible for or declining cisplatin-based chemotherapy.
Participants were randomly assigned in a 1:1 ratio to receive either:
- Perioperative enfortumab vedotin plus pembrolizumab followed by surgery, or
- Surgery alone (control group).
The neoadjuvant regimen consisted of 3 cycles of enfortumab vedotin (1.25 mg/kg on days 1 and 8 of a 3-week cycle) plus pembrolizumab (200 mg on day 1 every 3 weeks), followed by radical cystectomy. Postoperatively, patients received 6 cycles of adjuvant enfortumab vedotin plus 14 cycles of adjuvant pembrolizumab, for a total of 9 enfortumab cycles and 17 pembrolizumab cycles.
The primary endpoint was event-free survival (EFS), defined as time from randomization to radiographic progression precluding surgery, unresectable disease, recurrence, or death. Key secondary endpoints included overall survival (OS) and pathological complete response (pCR; defined as T0N0 at surgery). Safety was also assessed.
Study Design
A total of 344 participants were randomized:
- 170 to the enfortumab vedotin–pembrolizumab group
- 174 to the surgery-alone control group
Median follow-up was 25.6 months (range, 11.8 to 53.7). Baseline characteristics reflected a frail, cisplatin-ineligible population:
- 75% were men
- 80% were ≥65 years of age
- 80% were cisplatin-ineligible according to Galsky criteria
- 75% had stage T3N0 or T4aN0 disease
- 10% had ECOG performance status 2
Creatinine clearance <60 ml/min was the most common reason for cisplatin ineligibility.
Surgery was performed in 87.6% of patients in the combination group and 89.7% in the control group, indicating that neoadjuvant therapy did not compromise surgical feasibility.
Results
Event-Free Survival
An event or death occurred in:
- 48 patients (28.2%) in the enfortumab vedotin–pembrolizumab group
- 95 patients (54.6%) in the control group
The event rate was 1.4 per 100 person-months with combination therapy versus 3.9 per 100 person-months in the control group.
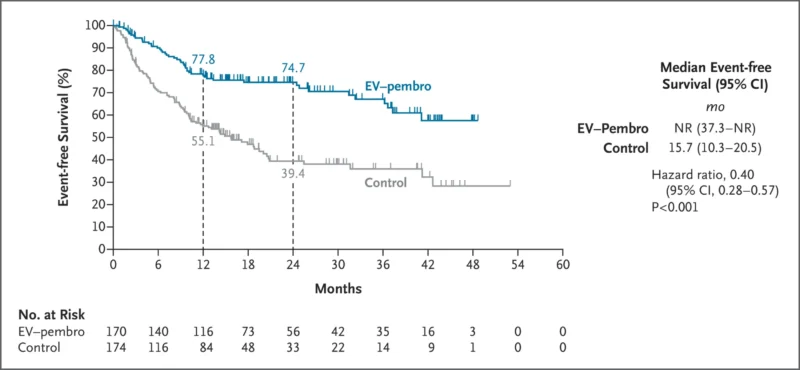
At 2 years:
- EFS was 74.7% (95% CI, 66.9–80.8) in the combination group
- EFS was 39.4% (95% CI, 31.0–47.8) in the control group
Hazard ratio (HR) for event or death: 0.40 (95% CI, 0.28–0.57; P<0.001).
Median EFS was not reached in the combination arm versus 15.7 months in the control arm.
Overall Survival
Deaths occurred in:
- 22.4% in the combination group
- 39.1% in the control group
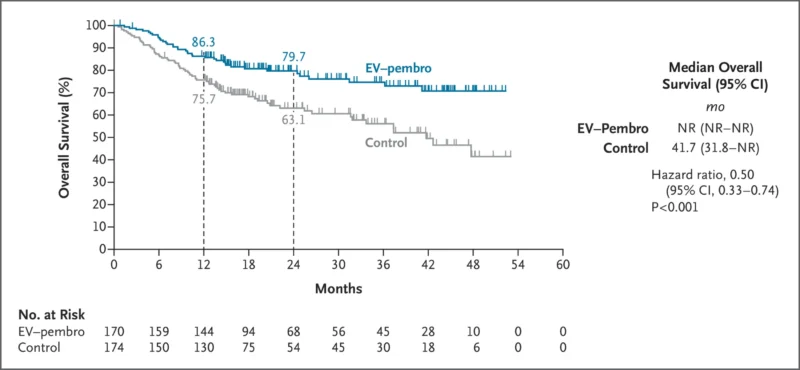
At 2 years:
- OS was 79.7% (95% CI, 72.5–85.3) with combination therapy
- OS was 63.1% (95% CI, 54.7–70.4) with surgery alone
HR for death: 0.50 (95% CI, 0.33–0.74; P<0.001).Median OS was not reached in the combination group and was 41.7 months in the control group.
Pathological Complete Response
Pathological complete response occurred in:
- 57.1% of patients in the enfortumab vedotin–pembrolizumab group
- 8.6% in the control group
Absolute difference: 48.3 percentage points (95% CI, 39.5–56.5; P<0.001).
Pathological downstaging (below pT2N0) occurred in 65.9% vs. 12.6%, respectively.
Adverse events of any grade occurred in:
- 100% of patients in the combination group (grade ≥3 in 71.3%)
- 64.8% in the control group (grade ≥3 in 45.9%)
Drug-related grade ≥3 adverse events occurred in 45.5% of patients receiving combination therapy.
Most common adverse events (any grade) in the combination group:
- Pruritus (47.3%)
- Alopecia (34.7%)
Most common grade ≥3 event:
- Urinary tract infection (12.0%)
Serious adverse events occurred in 58.1% of patients in the combination arm.Treatment-related discontinuation occurred in 37.1% of patients. Adverse-event–related deaths occurred in 7.8% of patients in the combination group and 5.7% in the control group, primarily during the perioperative period.
Immune-mediated adverse events included hypothyroidism (14.4%) and severe skin reactions (13.8%). Peripheral neuropathy occurred in 36.5%, mostly grade ≤2.
Key Findings
- Perioperative enfortumab vedotin plus pembrolizumab significantly improved event-free survival (HR 0.40) and overall survival (HR 0.50).
- Two-year EFS improved from 39.4% to 74.7%.
- Two-year OS improved from 63.1% to 79.7%.
- Pathological complete response increased from 8.6% to 57.1%.
- Surgery rates were similar between groups, suggesting feasibility of neoadjuvant therapy.
- Toxicity was higher with combination therapy but consistent with known safety profiles.
Conclusion
The phase 3 KEYNOTE-905/EV-303 trial demonstrates that perioperative enfortumab vedotin plus pembrolizumab significantly improves event-free survival, overall survival, and pathological complete response compared with surgery alone in patients with muscle-invasive bladder cancer who are ineligible for or decline cisplatin-based chemotherapy.
With a 60% relative reduction in risk of recurrence or death and a 50% reduction in risk of death, this regimen establishes a new evidence-based perioperative option for this vulnerable population. Regulatory approval by the U.S. Food and Drug Administration has followed, and the combination now represents a major advance in the management of cisplatin-ineligible muscle-invasive bladder cancer.


