KEYNOTE-905/EV-303 (NCT03924895), a phase 3, open-label, randomized trial, evaluated perioperative enfortumab vedotin (EV) plus pembrolizumab (pembro) compared with surgery alone in patients with muscle-invasive bladder cancer (MIBC) who were ineligible for or declined cisplatin-based chemotherapy.
Presented by Prof. Christof Vulsteke at the ESMO Congress 2025, the study demonstrated that adding EV + pembro to radical cystectomy with pelvic lymph node dissection resulted in significant improvements in event-free survival (EFS), overall survival (OS), and pathologic complete response (pCR). These findings mark a major advance for cisplatin-ineligible MIBC, establishing a potential new perioperative standard of care.
Background
Radical cystectomy with pelvic lymph node dissection (RC + PLND) remains the standard of care for patients with muscle-invasive bladder cancer. However, nearly half of these patients are ineligible for cisplatin-based chemotherapy due to renal dysfunction, frailty, or comorbidities, leaving a substantial population without effective perioperative options.
Previous studies have shown limited benefit from surgery alone in this setting, underscoring the unmet need for alternative strategies. Enfortumab vedotin, an antibody–drug conjugate targeting Nectin-4, combined with pembrolizumab, has demonstrated potent antitumor activity and a favorable safety profile in metastatic urothelial cancer, providing a strong rationale for evaluation in the perioperative context.
Methods
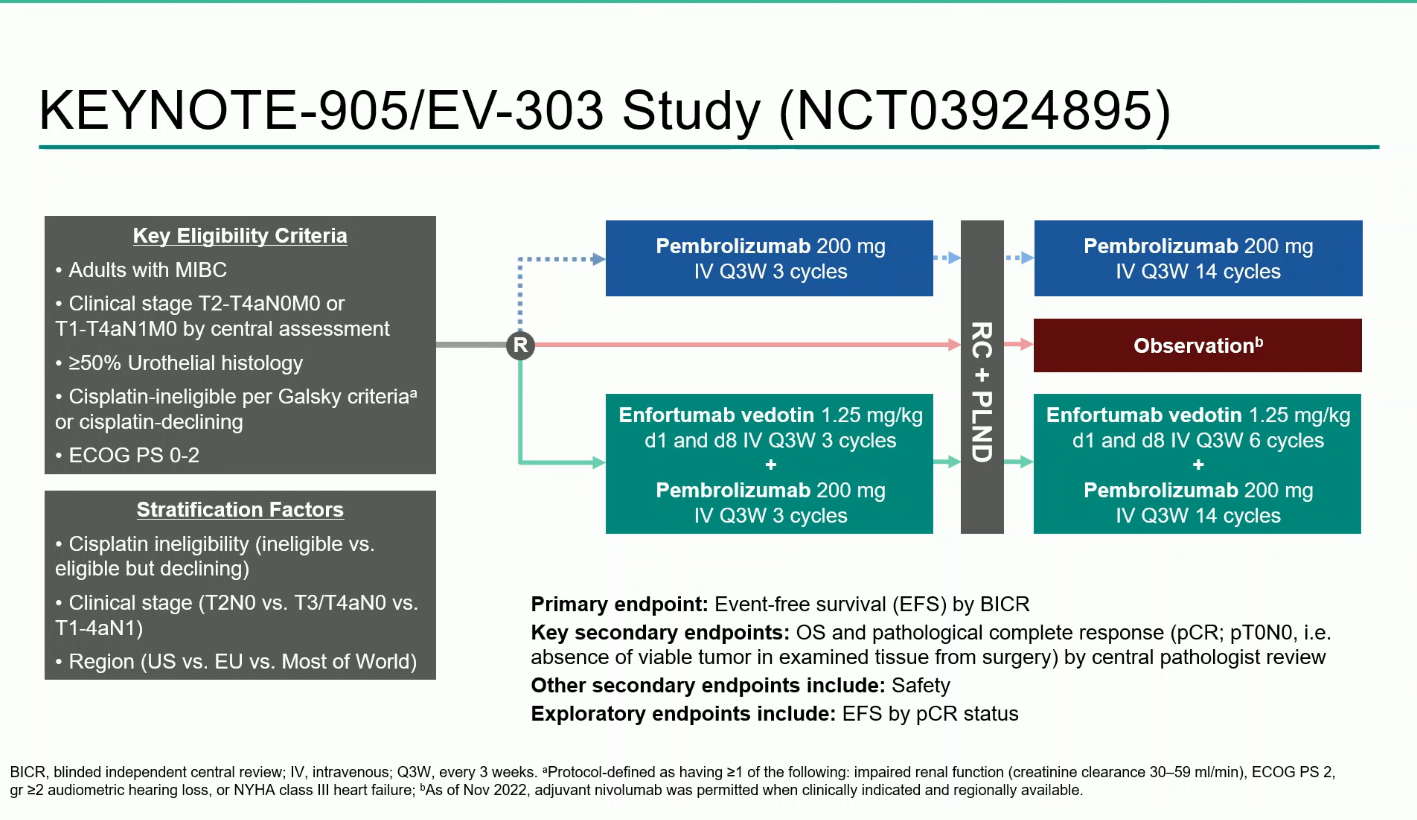
KEYNOTE-905/EV-303 is a randomized, open-label, phase 3 trial that enrolled adults with MIBC (T2–T4aN0M0 or T1–T4aN1M0) who were cisplatin-ineligible per Galsky criteria or declined cisplatin.
Participants were randomized 1:1 to receive:
- EV + pembro arm: Three cycles of EV (1.25 mg/kg on days 1 and 8) plus pembrolizumab (200 mg every three weeks) before RC + PLND, followed by six additional EV cycles and 14 cycles of pembrolizumab postoperatively.
- Control arm: RC + PLND alone, with adjuvant nivolumab permitted when clinically indicated.
The primary endpoint was event-free survival (EFS) by blinded independent central review (BICR).Key secondary endpoints included overall survival (OS), pathologic complete response (pCR), and safety.
Timeline of study development:
- The study initiated in 2019 with two treatment arms—perioperative pembrolizumab + RC + PLND vs RC + PLND alone—randomized 1:1.
- In 2020, a third arm was added, introducing perioperative EV + pembro + RC + PLND, changing the randomization to 1:1:1.
- By 2022, the design evolved: the pembrolizumab-alone arm was discontinued, and randomization was updated to 1:1 between EV + pembro + RC + PLND (EV + pembro arm) and RC + PLND (control arm).
- The inclusion criteria were expanded to include patients who were cisplatin-eligible but declined cisplatin, broadening the real-world relevance of the study population.
- Adjuvant nivolumab was permitted in the control arm per local guidelines.
Results
A total of 344 participants were randomized between December 2020 and June 2024 (170 to EV + pembro; 174 to control). Over 80% were cisplatin-ineligible, and the median follow-up was 25.6 months (range 11.8–53.7).The combination regimen produced statistically significant and clinically meaningful improvements across all efficacy endpoints:
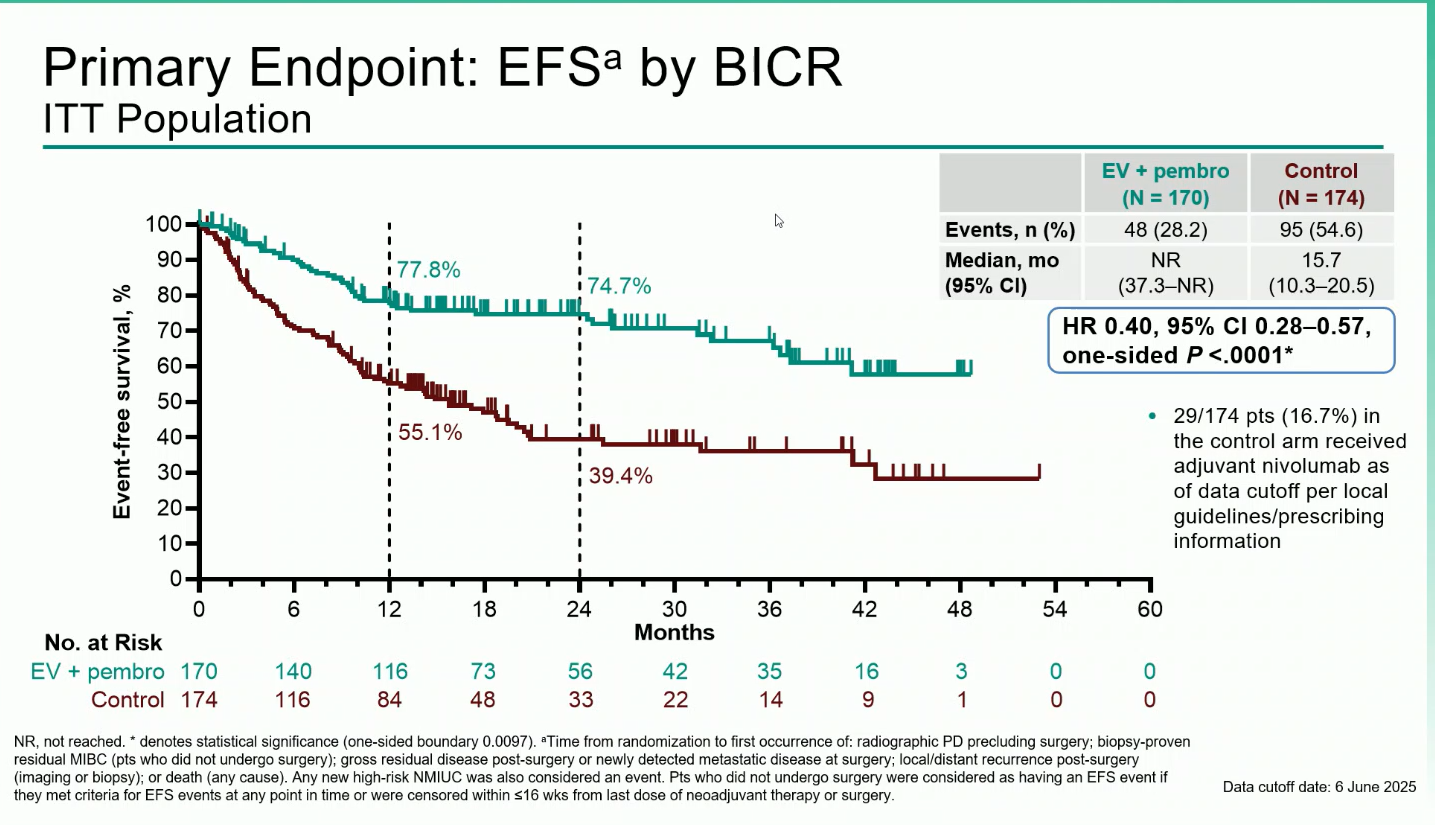
- Event-Free Survival (EFS): Median EFS was not reached with EV + pembro versus 15.7 months with control (HR 0.40; 95% CI 0.28–0.57; P<0.0001). At one years, EFS rates were 77.8% vs 55.1%, respectively and at two years, 74,7% vs 39,4%
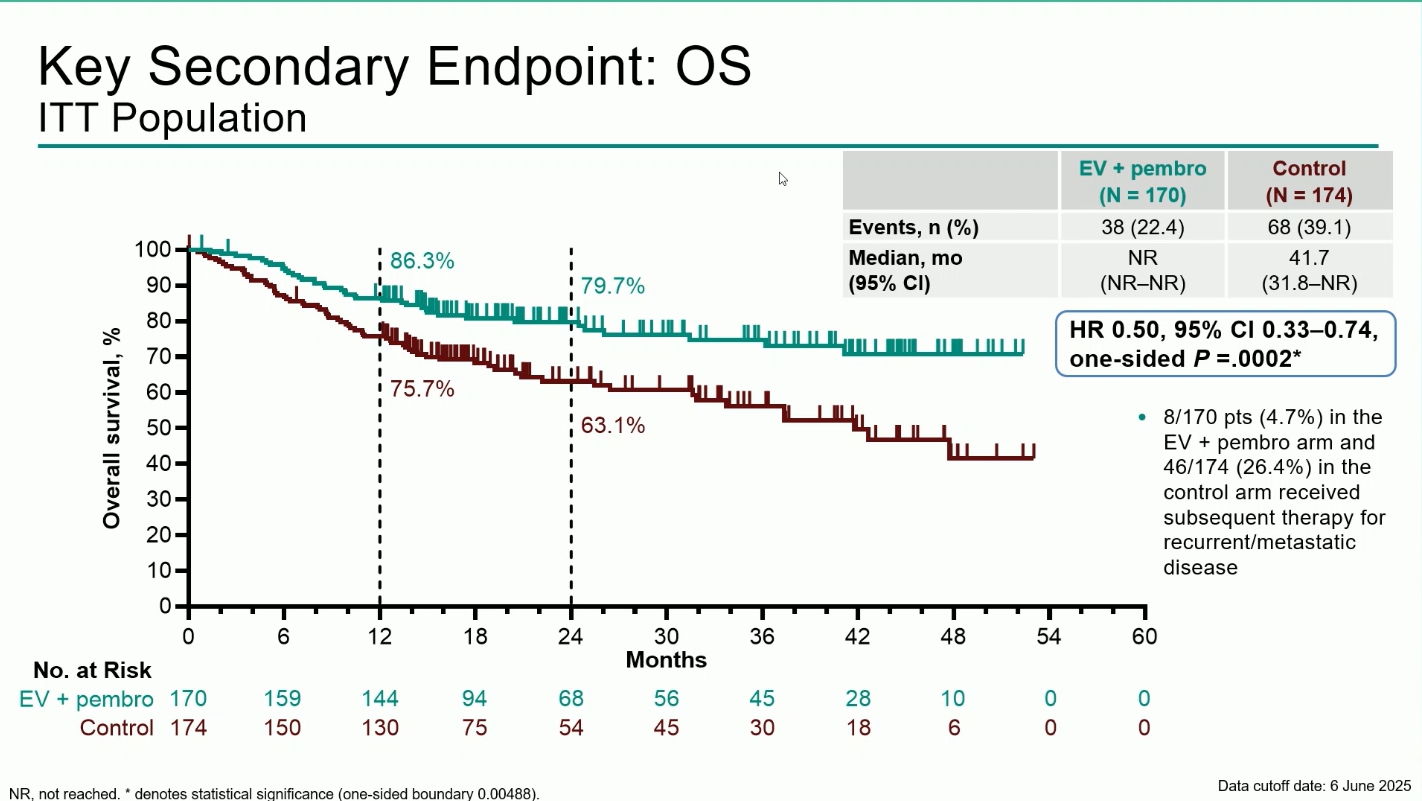
- Overall Survival (OS): Median OS was not reached with EV + pembro versus 41.7 months with control (HR 0.50; 95% CI 0.33–0.74; P=0.0002). The 12-month OS rates were 86.3% vs 75.7%, and the 24-months OS rates were 79,7% vs 63,1%
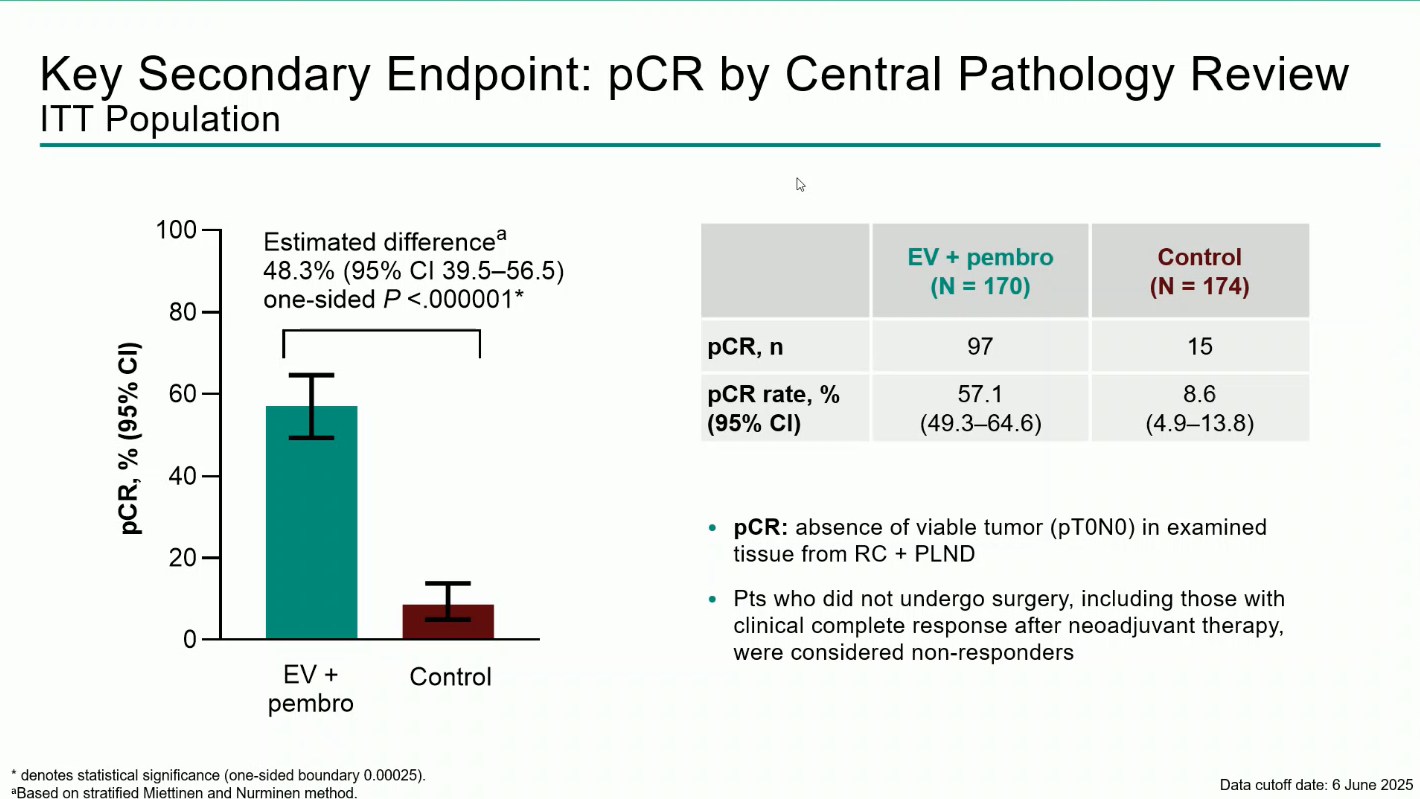
- Pathologic Complete Response (pCR): 57.1% with EV + pembro vs 8.6% with control, an absolute difference of 48.3% (95% CI 39.5–56.5; P<0.0001).
Safety:
Treatment-emergent adverse events (TEAEs) occurred in nearly all patients (100% in EV + pembro vs 64.8% in control). Grade ≥3 events were reported in 71.3% and 45.9%, respectively. The most frequent grade ≥3 adverse events of special interest were severe skin reactions (11.4%) related to pembrolizumab and cutaneous toxicity (10.8%) associated with enfortumab vedotin.
Despite the high incidence of AEs, the safety profile remained manageable and consistent with prior studies, and no new safety signals were identified.
Conclusions
The KEYNOTE-905/EV-303 trial demonstrated that adding perioperative enfortumab vedotin plus pembrolizumab to standard surgery significantly improved event-free survival, overall survival, and pathologic complete response rates in patients with cisplatin-ineligible MIBC.
These findings establish EV + pembrolizumab as the first perioperative regimen to improve outcomes versus RC + PLND alone in this population, offering a potential new standard of care.
You can read the full abstract here.


