JCOG0603 Trial was a randomized phase II/III trial designed to clarify the role of adjuvant systemic therapy after curative-intent hepatectomy in patients with resectable colorectal liver-only metastases, comparing postoperative modified FOLFOX6 with surgery alone. While earlier analyses demonstrated a meaningful improvement in disease-free survival with adjuvant chemotherapy, long-term follow-up was required to determine whether this benefit translated into improved overall survival and durable clinical advantage in this high-risk population.
Title: Randomized Phase II/III Trial Comparing Hepatectomy, Followed by mFOLFOX6 With Hepatectomy Alone for Liver Metastasis From Colorectal Cancer: Long-Term Results of JCOG0603
Authors: Yukihide Kanemitsu, MD, Yasuhiro Shimizu, MD, PhD, Junki Mizusawa, PhD, Yoshitaka Inaba, MD, PhD, Shunsuke Tsukamoto, MD, Atsuo Takashima, MD, Masayuki Ohue, MD, PhD, Koji Komori, MD, PhD, Akio Shiomi, MD, Manabu Shiozawa, MD, PhD, Yusuke Suwa, MD, PhD , Takeshi Suto, MD, Yusuke Kinugasa, MD, PhD, Yasumasa Takii, MD, Hiroyuki Bando, MD, PhD, Takaya Kobatake, MD, Masafumi Inomata, MD, PhD, Yasuhiro Shimada, MD, Hiroshi Katayama, MD, and Haruhiko Fukuda, MD.
Background
Hepatectomy remains the only potentially curative treatment for patients with colorectal liver-only metastases (CRLM), yet recurrence rates after resection remain high, reaching 70–80% in historical series. Approximately half of these recurrences occur within the liver, underscoring the challenge of achieving durable disease control. The role of perioperative or adjuvant systemic chemotherapy in this setting has remained controversial, particularly given the lack of consistent overall survival (OS) benefit across trials. Prior studies, including the EORTC 40983 trial, demonstrated improvements in progression-free survival with perioperative FOLFOX but failed to show OS benefit.
The Japanese Clinical Oncology Group (JCOG) designed the JCOG0603 to evaluate whether adjuvant modified FOLFOX6 (mFOLFOX6) after curative hepatectomy could improve long-term outcomes compared with surgery alone in patients with resectable CRLM.
Methods
Eligible patients had histologically confirmed colorectal adenocarcinoma with liver-only metastases deemed completely resectable. Importantly, there was no upper limit on the number of liver metastases, broadening applicability to real-world surgical practice. Patients were randomized in a 1:1 ratio to either postoperative observation after hepatectomy or adjuvant mFOLFOX6. Chemotherapy was initiated 56–84 days after surgery and administered every two weeks for up to 12 cycles, unless disease recurrence or unacceptable toxicity occurred.
Disease-free survival (DFS) was the primary endpoint for the phase III component, while OS was a predefined secondary endpoint. Survival assessments followed a structured schedule extending beyond five years, allowing for robust long-term outcome evaluation.
Study Design
JCOG0603 was a multicenter, randomized phase II/III trial conducted across 46 hospitals in Japan. Between March 2007 and January 2019, a total of 300 patients were enrolled and randomized: 151 to adjuvant mFOLFOX6 and 149 to hepatectomy alone. The study was conducted in accordance with institutional review board approvals, and all participants provided written informed consent. Survival analyses were performed on an intention-to-treat basis using Kaplan–Meier methodology, with hazard ratios estimated via Cox proportional hazards models.
Results
Baseline demographic and disease characteristics were well balanced between treatment arms. Median age was 63–65 years, and more than 90% of patients had three or fewer liver metastases, with lesions smaller than 5 cm in the majority of cases. Prior chemotherapy was slightly more frequent in the hepatectomy-alone arm (26% vs 17%). Molecular data, including RAS, BRAF, and MSI/MMR status, were available in only a minority of patients, limiting biologic stratification.
After a median follow-up of 7.7 years among disease-free survivors, 54 patients (35.8%) in the adjuvant chemotherapy arm and 51 patients (34.2%) in the hepatectomy-alone arm had died. There was no statistically significant difference in OS between the two strategies. The hazard ratio for OS was 1.07 (95% CI, 0.73–1.57; P = .736), indicating no survival advantage with adjuvant mFOLFOX6.
Five-year OS was 73.4% (95% CI, 65.5–79.7) in the adjuvant chemotherapy arm versus 80.1% (95% CI, 72.6–85.7) in the hepatectomy-alone arm. At seven years, OS remained comparable at 69.4% versus 72.4%, respectively. These results confirm that the early DFS advantage observed with adjuvant chemotherapy did not translate into a long-term survival benefit.
In contrast to OS, DFS remained significantly improved with adjuvant mFOLFOX6. The five-year DFS rate was 49.7% (95% CI, 41.5–57.3) in the chemotherapy arm compared with 40.5% (95% CI, 32.5–48.3) in the surgery-alone arm. The hazard ratio for DFS was 0.72 (95% CI, 0.54–0.97), reflecting a 28% relative reduction in the risk of recurrence or death. This DFS benefit was consistent with earlier reports from the trial.
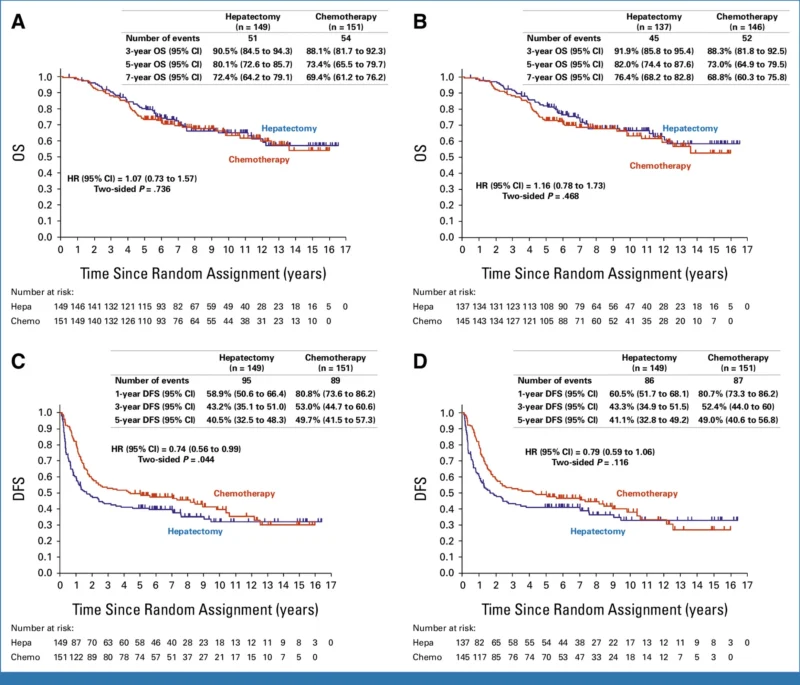
Recurrence occurred in 48% of patients receiving adjuvant chemotherapy and 56% of those managed with surgery alone. Remnant liver recurrence was more common in the hepatectomy-alone arm (61% vs 47%), whereas lung recurrence rates were similar between groups. Importantly, more patients in the hepatectomy-alone arm underwent repeat surgical resection after recurrence, and a higher proportion achieved R0–1 resections during post-trial surgery (60% vs 50%). Differences in post-recurrence systemic therapy were also observed, with greater use of oxaliplatin-based regimens in the hepatectomy-alone arm, potentially influencing long-term survival outcomes.
One treatment-related death occurred in the adjuvant chemotherapy arm, attributed to protocol therapy toxicity, while one patient in the surgery-alone arm died due to complications from post-protocol treatment. High rates of grade ≥3 adverse events, including neutropenia, were previously reported with adjuvant mFOLFOX6, raising concerns about tolerability and quality of life in the absence of OS benefit.
Key Findings
- Adjuvant mFOLFOX6 after hepatectomy improved disease-free survival (DFS) in resectable colorectal liver-only metastases.
- Overall survival (OS was not improved) compared with hepatectomy alone.
- DFS was not a reliable surrogate for OS in this clinical setting on long-term follow-up.
- Post-recurrence management differed between arms (repeat surgery and systemic therapies), which likely contributed to OS curves converging over time.
Conclusion
The long-term results of JCOG0603 demonstrate that adjuvant mFOLFOX6 following hepatectomy for colorectal liver-only metastases improves disease-free survival without improving overall survival. These findings align with prior perioperative chemotherapy trials and challenge the assumption that delayed recurrence necessarily translates into prolonged life. In the absence of a survival advantage and given the substantial toxicity associated with oxaliplatin-based chemotherapy, routine postoperative mFOLFOX6 cannot be universally recommended for resectable CRLM.
Future studies should focus on identifying biologically defined subgroups that may derive meaningful benefit from adjuvant therapy and on integrating predictive biomarkers to personalize postoperative treatment strategies.