The INTERACT-ION trial addresses the growing clinical challenge of squamous cell anal carcinoma (SCAC), a rare but increasingly diagnosed malignancy with age-adjusted incidence rising by more than 2% annually over the past decade. In patients with locally advanced, stage 3 disease, the current standard of care—concurrent chemoradiotherapy with mitomycin and fluorouracil or capecitabine—can provide durable tumour control, yet nearly 40% still experience recurrence or persistent disease. Treatment-related toxicity also remains substantial, with some patients requiring colostomy due to therapy-associated complications.
As immune checkpoint inhibitors have demonstrated activity in recurrent or metastatic SCAC, there is growing interest in integrating immunotherapy earlier in the treatment pathway. The INTERACT-ION phase 2 trial evaluated a novel induction strategy combining the PD-1 inhibitor ezabenlimab with modified docetaxel, cisplatin, and fluorouracil (mDCF), followed by response-adapted involved-node chemoradiotherapy (INRT) and ezabenlimab maintenance, aiming to improve complete response rates and long-term outcomes while potentially reducing treatment-related toxicity in patients with treatment-naive stage 3 SCAC.
Title: Ezabenlimab and induction chemotherapy followed by adaptive chemoradiotherapy in patients with stage 3 squamous cell anal carcinoma (INTERACT-ION)
Authors: Stefano Kim, MD, Jihane Boustani, MD, Soledad Iseas, MD, Dewi Vernerey, PhD, Ludovic Evesque, MD, Prof Laurent Quero, MD, Prof Francois Ghiringhelli, MD, Clélia Coutzac, MD, Prof Olivier Bouché, MD, Benoist Chibaudel, MD, Chloé Vernet, MD, Angélique Vienot, MD, Prof David Tougeron, MD, Prof Thierry Nguyen, MD, Magali Rebucci-Peixoto, PhD, Aurélia Meurisse, BScd, Sarah Chennoufi, MD, Christophe Maritaz, PharmD, Christophe Borg, MD
Published in Lancet Oncology, November 2025
Background
Locally advanced squamous cell anal carcinoma (SCAC) is usually treated with concurrent chemoradiotherapy using mitomycin plus fluorouracil or capecitabine with pelvic radiotherapy. Despite being the standard of care, around 40% of patients with stage 3 disease experience persistent or recurrent cancer, and treatment can lead to substantial toxicity, including treatment-related colostomy in up to 20–30% of cases in some series.
At the same time, anti–PD-1 agents such as nivolumab and pembrolizumab have shown meaningful activity in recurrent or metastatic SCAC but modest response rates when used alone. The INTERACT-ION phase 2 trial investigated whether combining the PD-1 inhibitor ezabenlimab with modified docetaxel, cisplatin, and fluorouracil (mDCF) as induction therapy, followed by personalised involved-node chemoradiotherapy (INRT) and ezabenlimab maintenance, could improve response rates and long-term outcomes in patients with treatment-naive stage 3 SCAC.
Methods
INTERACT-ION was an open-label, single-arm, phase 2 study conducted across ten French hospitals and university centres. Eligible patients were adults (≥18 years) with newly diagnosed, locally advanced, histologically confirmed stage 3 SCAC (TxN1 or T4N0, AJCC 8th edition), ECOG performance status 0–1, and adequate haematologic and organ function. Patients with prior pelvic radiotherapy, prior chemotherapy, antitumour immunotherapy, or metastatic disease were excluded. People living with HIV were eligible only if their CD4 count was ≥400/mm³.
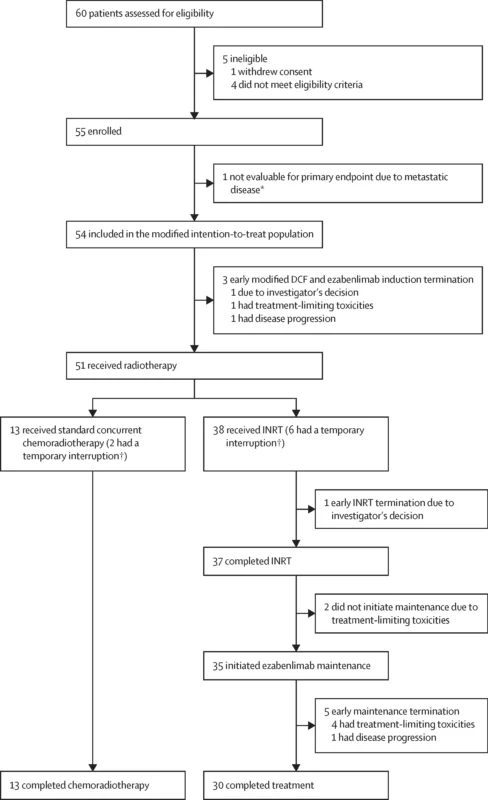
All enrolled patients received induction mDCF plus ezabenlimab. Activity was assessed in the modified intention-to-treat (mITT) population, defined as all evaluable patients who received at least one cycle of treatment and were assessable for the primary endpoint. Safety was assessed in all patients who received at least one study medication (safety population).
The primary endpoint was clinical complete response at week 40, defined as the proportion of patients alive with no clinical or radiologic evidence of residual disease (by MRI and/or CT), with a two-sided 90% CI lower bound >65%. Secondary endpoints included pathological complete or near-complete response after induction, biological complete response assessed by HPV circulating tumour DNA (ctDNA), objective response rate, disease control at 8 weeks, progression-free survival (PFS), disease-free survival (DFS), overall survival (OS), safety, and health-related quality of life (HRQOL).
Study Design
Patients received induction therapy with mDCF every 2 weeks for four cycles and Ezabenlimab 240 mg intravenously every 3 weeks for three cycles
- Docetaxel 40 mg/m² on day 1
- Cisplatin 40 mg/m² on day 1
- Fluorouracil 1200 mg/m² on days 1–2
- Ezabenlimab 240 mg intravenously every 3 weeks for three cycles
At week 8, response was assessed with RECIST v1.1 imaging (CT and MRI), tumour biopsy, and HPV ctDNA. Patients without disease progression received two additional cycles of mDCF and one additional cycle of ezabenlimab.
After these additional cycles, patients who achieved a major radiologic response (≥30% tumour reduction), a pathological complete or near-complete response (<10% viable tumour cells on biopsy), anda biological complete response (clearance of HPV ctDNA from detectable >20 copies/mL at baseline to undetectable) were assigned to intensity-modulated involved-node radiotherapy (INRT) with concurrent capecitabine and mitomycin, followed by seven cycles of ezabenlimab maintenance (240 mg every 3 weeks).
Patients who did not meet these criteria received standard concurrent chemoradiotherapy (full pelvic fields) with capecitabine and mitomycin. All patients were followed until the end of the trial, with survival assessed using Kaplan–Meier methods and HRQOL measured using the EORTC QLQ-C30 questionnaire.
Results
Between Jan 4, 2022, and Nov 20, 2023, 60 patients were screened, 55 enrolled (safety population), and 54 were evaluable for the primary endpoint (mITT population). Median age was 63.9 years (IQR 57.1–72.3); 76% were female. Disease burden was high: 80% had T3–T4 tumours and 76% had nodal involvement (N1a–c), including 4% with M1a lymph node disease. Baseline HPV ctDNA was positive in approximately 75% of patients.
Induction therapy was delivered as planned in most patients: 53/54 (98%) completed the first four cycles of mDCF plus ezabenlimab, and 51/53 (96%) received the additional two cycles. Median dose intensity was high for all agents (around 98% for docetaxel, cisplatin, and fluorouracil).
After induction:
- Objective response rate: 49/53 patients (93%)
- Complete response: 13 (25%)
- Partial response: 36 (68%)
- Stable disease: 3 (6%)
- Progressive disease: 1 (2%)
- Disease control rate at 8 weeks: 98% (52/53)
- Pathological complete or near-complete response: 41/49 (84%)
- Biological complete response (HPV ctDNA clearance): 36/40 (90%)
Based on these responses, 38/51 (75%) patients proceeded to INRT, while 13/51 (26%) received standard concurrent chemoradiotherapy.
At week 40 (primary endpoint):
- Clinical complete response in mITT: 42/54 patients (77.8%, 90% CI 66.5–86.7), meeting the predefined success criterion (lower CI >65%)
- INRT group: 86.8% clinical complete response (33 patients)
- Concurrent chemoradiotherapy: 69.2% clinical complete response (9 patients)
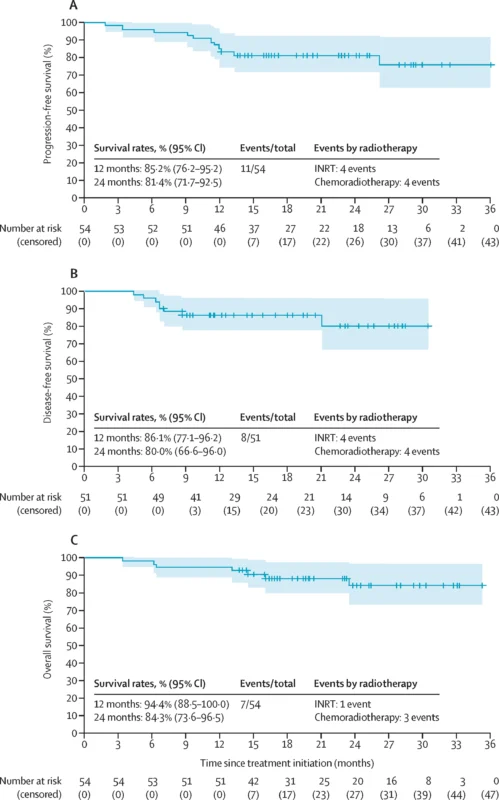
At a median follow-up of 23.0 months (95% CI 16.5–29.1), survival outcomes were encouraging, with medians not yet reached:
- PFS at was 12 months: 85.2% and at 24 months: 81.4%
- DFS (post-radiotherapy) at 12 months: 86.1%, at 24 months: 80.0%
- OS: at 12 months: 94.4% and at 24 months: 84.3%
Outcomes were numerically better in the INRT group, with 24-month PFS of 89.4% and OS of 97.4%, compared with lower PFS and OS in the standard chemoradiotherapy group. Only 3 patients (6%) required definitive colostomy during follow-up.
Key Findings
- Nearly all patients with induction ezabenlimab + mDCF achieved disease control at 8 weeks, with a 93% objective response rate.Pathological complete/near-complete response was 84%, and biological complete response based on HPV ctDNA clearance reached 90%.
- Primary endpoint achieved. Clinical complete response at 40 weeks was 77.8% (90% CI 66.5–86.7), exceeding the prespecified threshold based on historical ACT II data.
- Three-quarters of patients were eligible for INRT, a more focused radiation approach sparing uninvolved nodal regions.
- Locoregional control appeared preserved, with most recurrences occurring outside conventional radiation fields.
- Despite a high tumour burden (80% T3–T4; 76% node-positive), 2-year PFS and DFS were around 80%, and 2-year OS exceeded 80%, comparing favourably with historical chemoradiotherapy cohorts.
Manageable safety profile
- During induction, grade ≥3 treatment-related adverse events occurred in 33% of patients, mainly neutropenia, diarrhoea, nausea, anaemia, and asthenia.
- During INRT, the most frequent grade ≥3 events were lymphopenia (42% grade 3, 3% grade 4), neutropenia, epithelitis, and anal inflammation.
- During standard chemoradiotherapy, grade ≥3 lymphopenia occurred in all patients.
- Immune-related toxicities were common but mostly low grade; grade ≥3 immune-related events occurred in 15%. No treatment-related deaths were reported.
- HRQOL scores for global health status and physical functioning improved from baseline and remained higher at 40 weeks and during follow-up.
Conclusion
The INTERACT-ION Trial phase 2 trial provides strong proof-of-concept that induction ezabenlimab plus mDCF, followed by HPV ctDNA- and biopsy-guided INRT and ezabenlimab maintenance, is a promising strategy for patients with treatment-naive stage 3 squamous cell anal carcinoma. The regimen delivered high rates of clinical, pathological, and biological complete response, encouraging 2-year progression-free and overall survival, and a low rate of definitive colostomy in a population with high tumour burden. The safety profile was manageable, and quality of life improved over time.
These findings support further evaluation of ezabenlimab + mDCF–based chemoradiotherapy in a randomised phase 3 setting against standard concurrent chemoradiotherapy to determine whether this approach should become a new treatment standard for locally advanced SCAC.
You can read the full article here.