Original Study Title: Nationwide Insights on Immunotherapy in a Low- and Middle-Income Country: Armenia’s Struggle for Equitable Cancer Care in an Out-of-Pocket System
Authors: Amalya Sargsyan MD, MSc, Gevorg Tamamyan MD, PhD, MSc, DSc, Arman Oganisian PhD, Davit Zohrabyan MD, Liana Safaryan MD, Armen Avagyan MD, Lilit Harutyunayn MD, PhD, Mariam Sargsyan MD, Martin Harutyunyan MD, Nune Karapetyan MD, MPH, Naira Janoyan MD, PhD, Levon Badalyan MD, PhD, Mariam Mailian MD, Haykuhi Geokchyan MD, PhD, Vardan Bardakhchyan PhD, Elen Baloyan, MD, Hasmik Petrosyan MD, PhD, Karine Soghomonyan MD, Anna Tadevosyan MD, PhD, Karen Bedirian PharmaD, MPH , Shushan Hovsepyan MD, Samvel Bardakhchyan, MD, PhD.
This study provides the first real-world, nationwide evidence on the use of immune checkpoint inhibitors (ICIs) in Armenia, a lower-middle-income country (LMIC) with no universal health insurance and where most patients face out-of-pocket costs or rely on black-market procurement of expensive therapies. Despite these financial barriers, ICIs demonstrated clinically meaningful survival outcomes, especially in biomarker-positive subgroups, highlighting both the promise and the inequities of immunotherapy in resource-limited settings.
Study Design and Methodology
This multicenter, retrospective cohort study included patients treated with at least one dose of an ICI between January 2017 and December 2023 across six oncology centers in Armenia. A total of 373 patients with histologically confirmed malignancies and ≥6 months follow-up were analyzed. Data included demographics, tumor characteristics, treatment details, and biomarkers (PD-L1, MMR, driver mutations). Survival was estimated by the Kaplan-Meier method, with log-rank tests and Cox models for subgroup analyses. A 6-month landmark analysis was conducted to address immortal time bias.
Key Findings
-
Cohort: 373 patients; 67% male; median age 61; 68% with stage IV disease.
-
Median Overall Survival (OS): 31 months.
-
Predictors of Poorer OS:
-
Liver metastases (19 vs 26 months, P = .027)
-
Smoking status (24 vs 31 months for smokers vs nonsmokers, P = .019)
-
-
Predictors of Improved OS:
High PD-L1 (≥50%): 40 vs 16 months, P = .025
Receiving >6 cycles of ICI: 45 vs 23 months, P < .001 (not significant in landmark analysis)
Discontinuation Drivers: Financial hardship and disease progression were the most common reasons.
Tumor-specific outcomes: Stage IV melanoma patients had the longest OS (113 months).
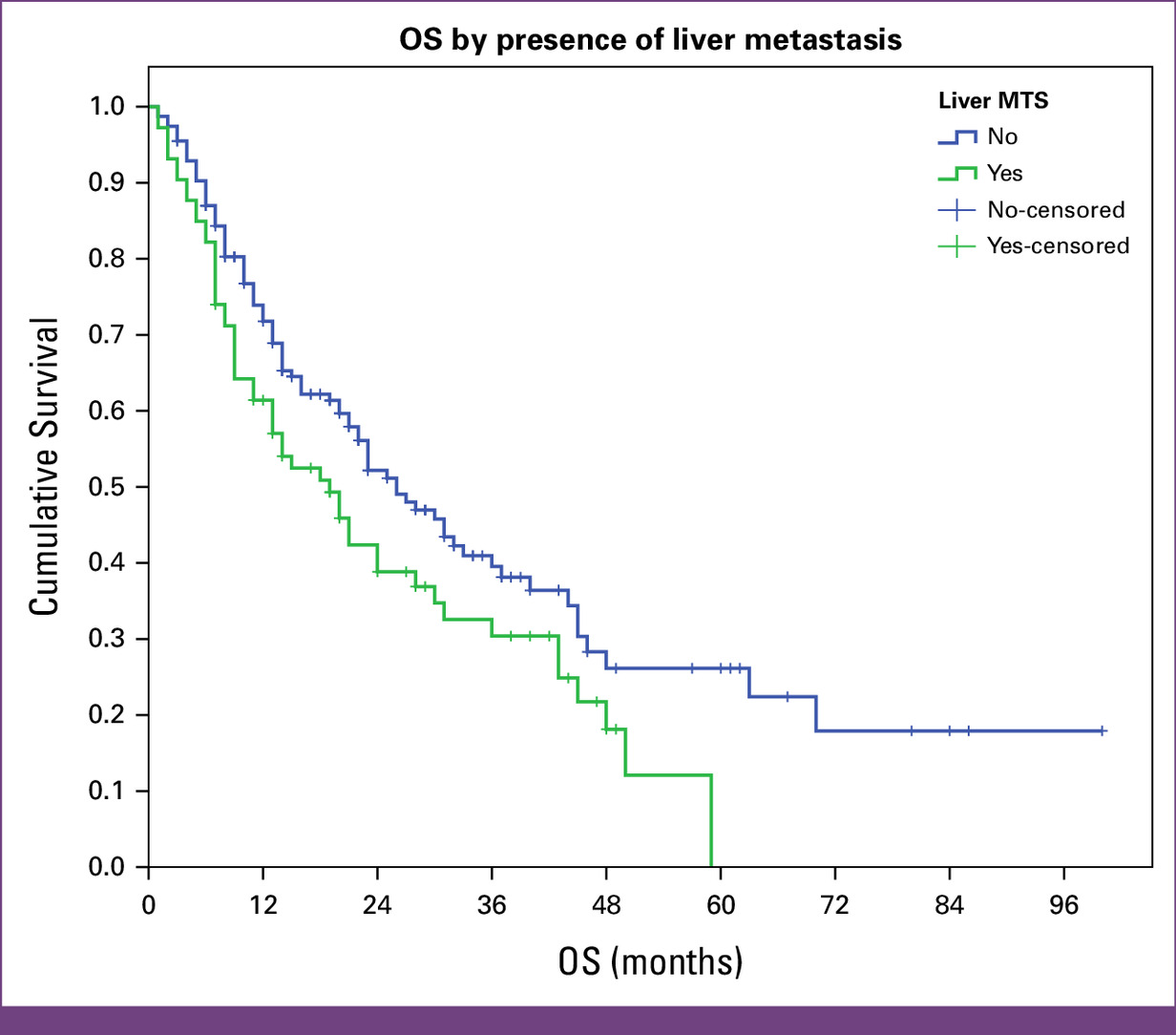
Context and Implications
Despite fragmented access, immunotherapy produced real-world survival benefits in Armenia, comparable with international trial data. These findings challenge the assumption that high-cost therapies cannot yield impact in LMICs.
Drawing on examples from India, China, and Brazil, Armenia could expand access through:
-
Pooled procurement and negotiated pricing
-
Partial reimbursement and tiered insurance
-
Prioritization of biomarker testing to allocate therapies efficiently
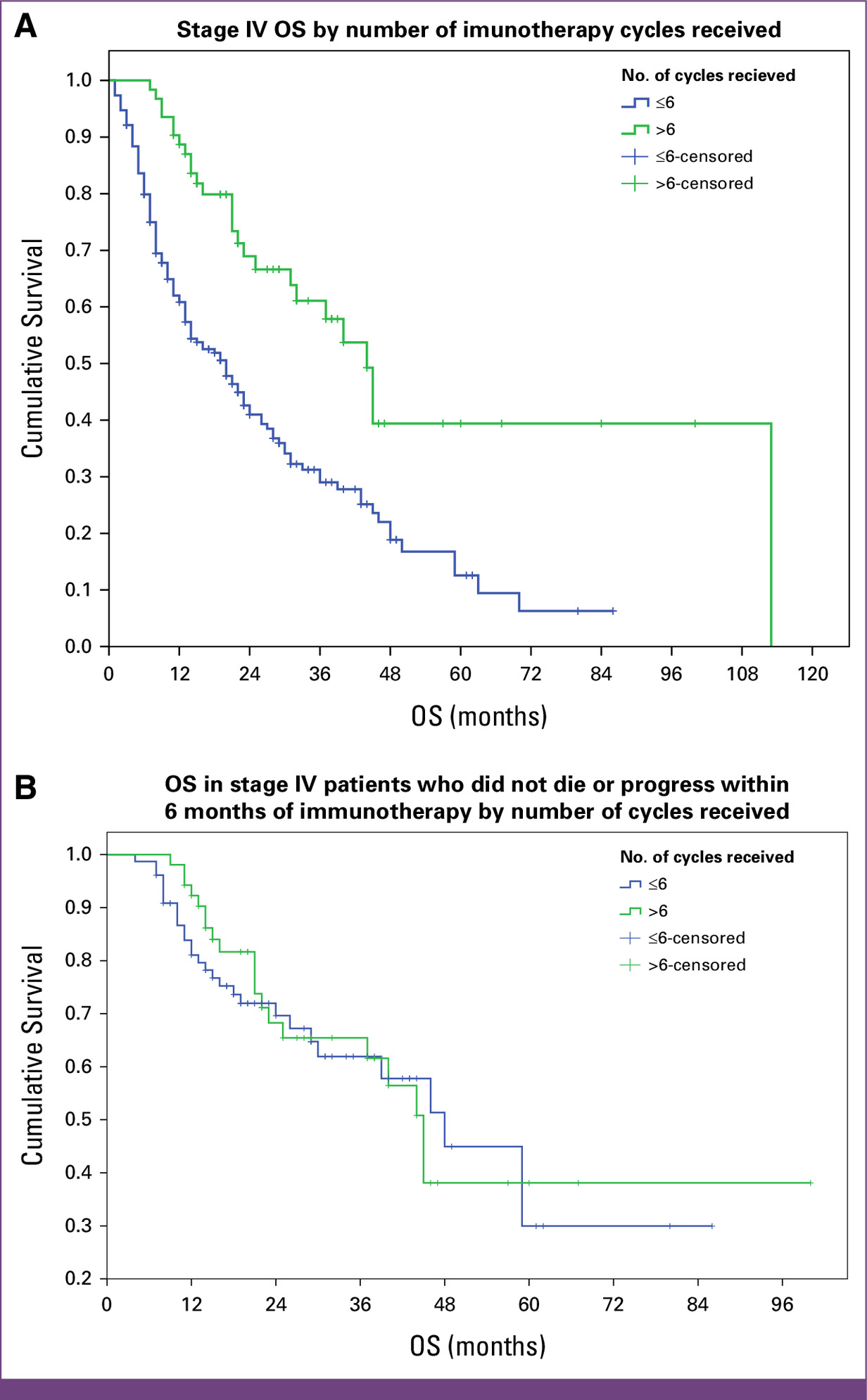
You Can Read All Article Here.
Written by Toma Oganezova, MD, Editor-in-Chief of OncoDaily IO


