Title: Pembrolizumab Plus Platinum-Based Chemotherapy for Patients With Advanced Penile Cancer – The Nonrandomized HERCULES (LACOG 0218) Clinical Trial
Authors: Fernando Cotait Maluf, PhD; Karine Trindade, MD; Daniel Preto, PhD; Murilo de Almeida Luz, MD; Patrícia Medeiros Milhomem Beato, MD; Diogo Assed Bastos, PhD; João Paulo Holanda Soares, MD; Victor Marcondes Lopes, MD; Luis Eduardo Werneck de Carvalho, PhD; David Queiroz Borges Muniz, MD; Douglas Jorge Racy, MD; Rafaela Gomes de Jesus, MSc; Taiane Francieli Rebelatto, MD; Gustavo Werutsky, PhD; Susan Halabi, PhD; Fernando Sabino Marques Monteiro, PhD; André Poisl Fay, PhD.
Journal: JAMA Oncology, Published Online September 18, 2025

Penile squamous cell carcinoma (PSCC) is a rare, aggressive cancer with poor outcomes in advanced stages. Standard systemic treatment has long relied on platinum-based chemotherapy, but survival gains have been modest and no new strategies have altered the clinical landscape in decades. Given the observed benefit of checkpoint inhibitors in HPV-related cancers such as cervical and head & neck SCC, investigators explored whether pembrolizumab combined with chemotherapy could improve outcomes in PSCC.
Study Design – HERCULES Trial (LACOG 0218)
The HERCULES trial (LACOG 0218) was a phase 2, single-arm, nonrandomized, multicenter study conducted across 11 centers in Brazil to evaluate pembrolizumab in combination with platinum-based chemotherapy for patients with advanced penile squamous cell carcinoma (PSCC). A total of 37 patients with metastatic, recurrent, or locally advanced disease not suitable for curative-intent therapy were enrolled.
Treatment consisted of pembrolizumab 200 mg given intravenously on day 1, combined with cisplatin 70 mg/m² IV on day 1 (or carboplatin AUC 5, at the investigator’s discretion), and fluorouracil 1000 mg/m²/day as a continuous infusion on days 1 through 4. This regimen was repeated every three weeks for six cycles, after which patients without disease progression continued pembrolizumab monotherapy every three weeks for up to 34 cycles.
The primary endpoint of the study was overall response rate (ORR) as assessed by RECIST v1.1. Secondary endpoints included clinical benefit rate, progression-free survival, overall survival, duration of response, safety, and health-related quality of life. Exploratory analyses also investigated potential biomarkers, including HPV status, PD-L1 expression, tumor mutational burden, and genomic alterations.
Results
The study enrolled 37 patients with a median age of 56 years (range, 30–76). Most had metastatic disease (65%), while 22% had recurrent and 14% had locally advanced PSCC.
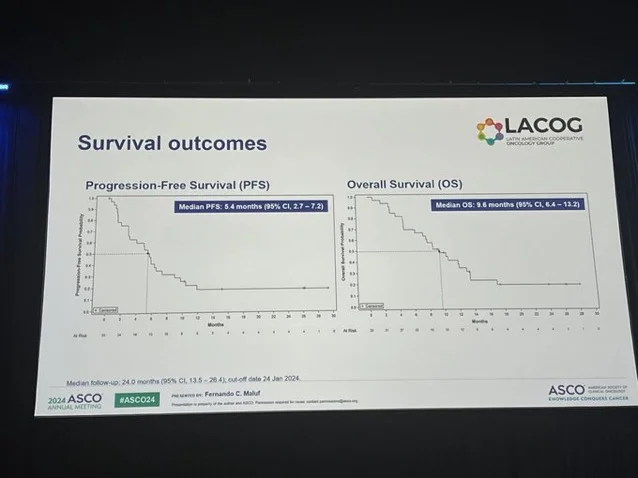
- Efficacy outcomes were encouraging. The overall response rate was 39.4% by investigator assessment and 42.4% by central review. Median progression-free survival was 5.4 months (95% CI, 2.7–7.2), and median overall survival reached 9.6 months (95% CI, 6.4–13.2). The clinical benefit rate was 45.5%, with several patients achieving durable disease control.
- Safety was manageable. Almost all patients experienced some adverse events (97%), and 51% had grade ≥3 events. The most common treatment-related toxicities were anemia (43%), nausea (43%), neutropenia (22%), and decreased appetite (24%). Immune-related adverse events occurred in 22% of patients, but only 5% were grade ≥3. Importantly, no treatment-related deaths were reported.
- Biomarker analyses suggested potential predictive trends. HPV-positive patients had higher response rates (56% vs 35%) and longer progression-free survival (7.6 vs 5.6 months). Patients with high tumor mutational burden (≥10 mut/Mb) showed particularly strong activity, with an ORR of 75% compared with 36% in TMB-low patients. In contrast, PD-L1 expression did not correlate with clinical outcomes.
Key Takeaways
- Pembrolizumab plus platinum-based chemotherapy showed meaningful clinical activity in advanced penile squamous cell carcinoma.
- Achieved ORR of ~40% and median OS of 9.6 months, exceeding historical outcomes with chemotherapy alone.
- Safety profile was manageable, with no treatment-related deaths and toxicities consistent with expectations.
HPV-positive and TMB-high patients demonstrated higher response rates and longer PFS. - Represents a promising first-line treatment option in a rare and aggressive cancer with limited therapeutic progress.


