The ESMO–ESTRO consensus addresses the growing clinical challenge of combining targeted systemic therapies with radiotherapy (RT), a scenario that is increasingly encountered in contemporary oncology practice. While such combinations may enhance tumor control, they also raise concerns regarding amplified toxicity due to overlapping effects on DNA repair, cell cycle regulation, and normal tissue radiosensitivity. In the absence of robust prospective safety data, clinical decision-making has largely relied on limited retrospective evidence. To bridge this gap, the European Society for Medical Oncology and the European Society for Radiotherapy and Oncology developed scenario-specific, consensus-based safety recommendations for the integration of RT with major targeted therapy classes.
Title: ESMO-ESTRO consensus statements on the safety of combining radiotherapy with CDK4/6, HER2, PARP, or mTOR inhibitors
Authors: Evert S.M. van Akena, Ajeet Kumar Gandhi, Sean M. O’Cathail, Gerben Borst, Jorge Barriuso, Emmanouil Fokas, Luis Castelo-Branco, Anne Hansen Ree, Evandro de Azambuja, Stephanie Kroeze, Ilaria Colombo, Antonin Levy, Carmen Criscitiello, Maximilian Niyazi, Nadia Harbeck, Ewa Szutowicz, Gabor Liposits, Marcel Verheija, Isabelle Ray-Coquard, Paolo Tarantino, Dario Trapani, Paulien Boot, Claus Belka, Dirk De Ruysschera, George Pentheroudakis, Corrie A.M. Marijnen, Florian Lordick, Umberto Ricardi, Diogo Martins-Branco, Arsela Prelaj, Monique C. de Jong, Bharti Devnani
Background
Radiotherapy (RT) remains a cornerstone of cancer treatment, with approximately 50% of patients receiving RT at some point during their disease course. In parallel, the use of targeted systemic therapies has expanded rapidly across tumor types and disease stages. As a result, clinicians increasingly encounter scenarios in which RT must be delivered concurrently or near-concurrently with targeted agents, including during palliation, oligometastatic treatment, or management of oligoprogression.
While combined treatment may improve tumor control, it can also amplify toxicity through overlapping biological mechanisms such as impaired DNA damage repair, altered cell cycle kinetics, or increased radiosensitivity of normal tissues. High-quality prospective data on the safety of these combinations are limited, and clinical practice has largely relied on small retrospective series or case reports. To address this unmet need, the European Society for Medical Oncology (ESMO) and the European Society for Radiotherapy and Oncology (ESTRO) conducted systematic literature reviews followed by a structured Delphi process to generate evidence-based, scenario-specific safety statements for combining RT with selected targeted therapies.
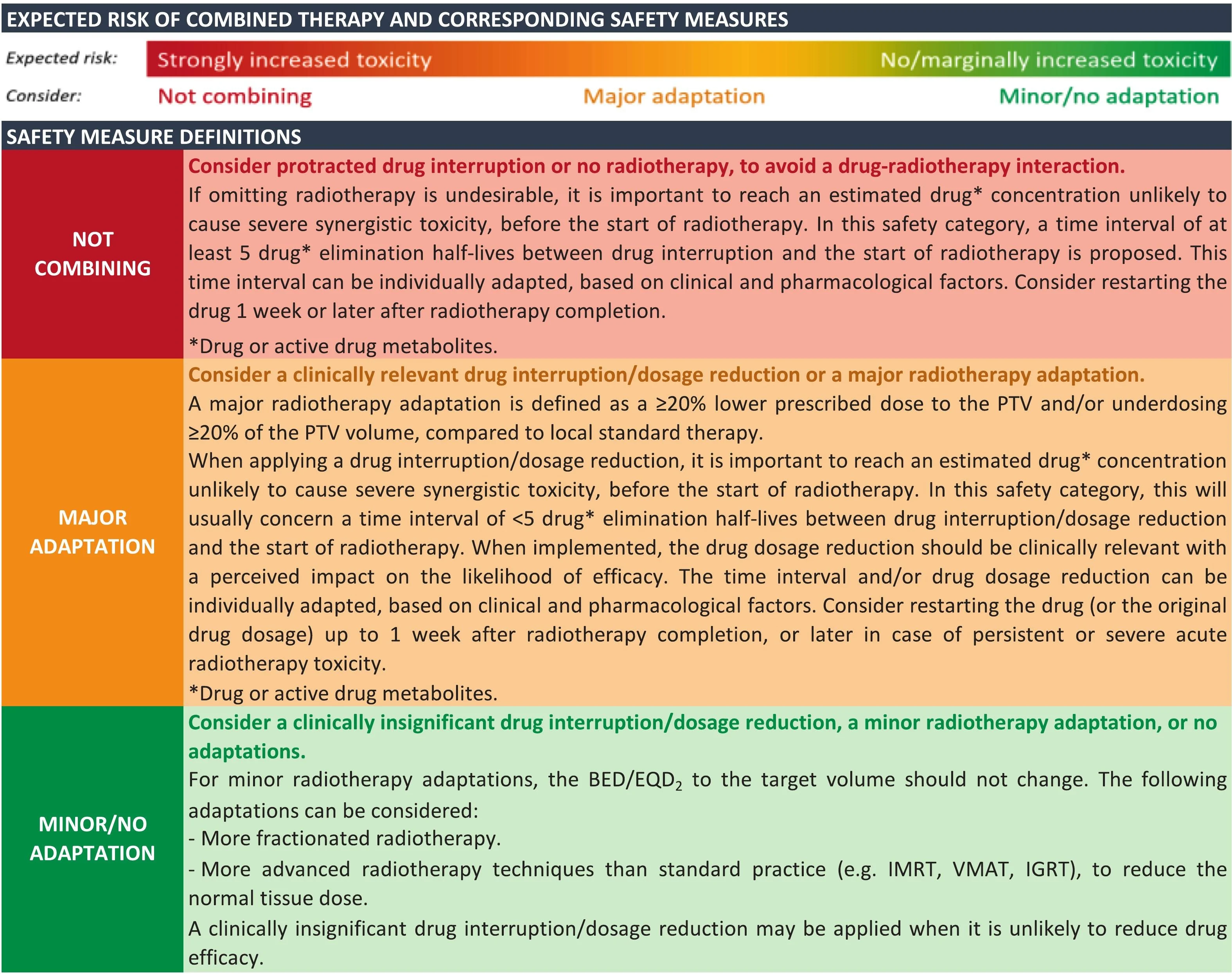
Methods
This initiative focused on four widely used drug classes: CDK4/6 inhibitors, anti-HER2 monoclonal antibodies, PARP inhibitors, and mTOR inhibitors. Systematic searches of Medline, Embase, and SCOPUS were performed, ultimately screening 1,341 records, of which 107 studies met inclusion criteria. Eligible studies reported toxicity outcomes when RT was administered concurrently with targeted therapy, defined as within five drug half-lives before RT or within two weeks after RT.
To ensure clinical relevance, toxicity data were analyzed according to six irradiated anatomical areas (skin, brain, head and neck, thorax, abdomen/pelvis, and musculoskeletal tissues) and three RT scenarios: low-dose palliative RT, high-dose conventionally fractionated RT, and high-dose stereotactic RT. A modified Delphi process was conducted with 20 international experts from ESMO and ESTRO, achieving a 90% response rate across two voting rounds. Consensus thresholds were predefined, with statements accepted at ≥90% agreement in round one or ≥75% agreement in round two. In total, 74 clinical scenarios were evaluated.
Results
For CDK4/6 inhibitors (palbociclib, ribociclib, abemaciclib), 18 studies were included. These agents arrest cells in the G1 phase, potentially impairing normal tissue recovery after RT. While most retrospective series reported acceptable toxicity with low-dose palliative RT, multiple case reports described unexpected grade 3 gastrointestinal toxicity, particularly when abdominal or pelvic organs were irradiated. The Delphi panel therefore recommended major treatment adaptation for most scenarios and advised against combining CDK4/6 inhibitors with high-dose RT to the head and neck, thorax, or abdomen/pelvis, each with 94–100% agreement. In contrast, minor or no adaptation was supported for low-dose palliative RT to skin and musculoskeletal sites.
For anti-HER2 monoclonal antibodies (trastuzumab and pertuzumab), 37 studies were included, largely reflecting experience in breast cancer. Overall, the expected additional RT-related toxicity was low. Across most irradiated areas and RT dose scenarios, the consensus favored minor or no treatment adaptation, with agreement rates frequently ≥94%. Cardiac toxicity remained a key consideration, as both RT and trastuzumab can impair cardiac function. Reported rates of grade ≥2 left ventricular ejection fraction decline ranged from 3% to 18% in concurrent treatment series. Increased caution was advised for high-dose RT to the abdomen/pelvis, high-dose stereotactic head and neck RT, and esophageal irradiation, where the panel recommended major adaptation due to limited data and potential for increased toxicity.
PARP inhibitors (including olaparib and veliparib) demonstrated the clearest radiosensitizing signal. From 17 included studies, largely early-phase trials, increased hematologic, pulmonary, and esophageal toxicity was consistently observed, even at PARP inhibitor doses well below standard monotherapy levels. Importantly, radiosensitization appeared possible despite drug interruption, due to persistent biological effects. As a result, the Delphi panel recommended major treatment adaptation for all 74 evaluated scenarios, regardless of irradiated site or RT dose, with agreement rates of 94–100% for most statements.
For mTOR inhibitors (everolimus, temsirolimus, sirolimus), 35 studies were included. While many combinations were feasible at reduced drug doses, overlapping toxicities—particularly mucositis, hematologic toxicity, and pneumonitis—were clinically relevant. The panel recommended minor or no adaptation for skin RT, low-dose palliative brain RT, and low-dose musculoskeletal RT. However, for high-dose conventionally fractionated thoracic RT, the consensus was not to combine mTOR inhibitors and RT (100% agreement) due to pneumonitis risk. For most other scenarios, major adaptation was advised.
Key findings
Across all drug classes, toxicity risk increased with higher RT dose, stereotactic techniques, and irradiation of mucosal or visceral organs. Anti-HER2 monoclonal antibodies were generally safe with RT, while CDK4/6 inhibitors, PARP inhibitors, and mTOR inhibitors required substantially greater caution. PARP inhibitors were associated with the most consistent need for treatment modification.
Key takeaway messages
These ESMO–ESTRO consensus statements provide pragmatic, scenario-specific guidance for clinicians facing complex RT–targeted therapy combinations. They highlight when concurrent treatment is reasonable, when modification is required, and when combinations should be avoided altogether.
Conclusion
Using systematic evidence review and a high-agreement Delphi process, ESMO and ESTRO developed 74 consensus safety statements addressing the combination of RT with major targeted therapy classes. While anti-HER2 monoclonal antibodies can usually be combined safely with RT, most scenarios involving CDK4/6, PARP, or mTOR inhibitors warrant caution or treatment adaptation. These recommendations offer an evidence-based framework to support safer multidisciplinary decision-making in daily oncology practice.
You can read the full article here.


