HER2-positive, or ERBB2-amplified, metastatic colorectal cancer (mCRC) represents a rare molecular subtype with growing therapeutic relevance as HER2-targeted strategies enter clinical practice. While ERBB2 amplification is a recognized mechanism of resistance to anti-EGFR antibodies, its prognostic impact in the first-line setting remains incompletely defined.
Using a large US-based real-world clinico-genomic database, this study evaluates the frequency of ERBB2 amplification and its association with survival outcomes in patients with mCRC treated with contemporary first-line chemotherapy regimens, providing important insights into an unmet clinical need in HER2-positive colorectal cancer.
Title: Survival outcomes of ERBB2-amplified metastatic colorectal cancer treated with first-line chemotherapy
Authors: Yuki Matsubara, Hideaki Bando, Yoshiaki Nakamura, Toshihiro Misumi, Dionne Ng, Eri Tajima, Harlan Pittell, Atsushi Ohtsu , Takayuki Yoshino
Published in Nature, December 2025
Background
HER2-positive, or ERBB2-amplified (ERBB2 amp+), metastatic colorectal cancer (mCRC) represents a small but clinically important molecular subgroup, accounting for approximately 2–4% of cases. ERBB2 amplification is well recognized as a mechanism of resistance to anti-EGFR antibodies, and HER2-targeted therapies are now established in later-line settings.
However, the optimal first-line strategy for ERBB2 amp+ mCRC remains unclear, particularly in patients who otherwise meet criteria for anti-EGFR therapy based on tumour sidedness and RAS/BRAF status. This study aimed to clarify the frequency and prognostic impact of ERBB2 amplification on real-world survival outcomes in patients receiving contemporary first-line chemotherapy-based treatments.
Methods
This retrospective analysis used the Flatiron Health–Foundation Medicine colorectal cancer clinico-genomic database (CGDB), which links deidentified real-world clinical data with tissue-based comprehensive genomic profiling. Eligible patients had stage IV or recurrent mCRC diagnosed between January 2012 and March 2022, were aged ≥18 years, had at least two documented clinical visits, and underwent tissue-based CGP within 90 days of metastatic diagnosis. Patients assessed only with liquid biopsy or with inadequate specimen quality were excluded.
ERBB2 amplification was defined as an ERBB2 copy number ≥ +3 relative to tumour base ploidy, consistent with global consensus criteria for HER2-positive mCRC. Survival analyses focused on real-world progression-free survival (rwPFS) and real-world overall survival (rwOS), measured from initiation of first-line therapy. Kaplan–Meier methods and Cox proportional hazards models were used, with adjustment for key clinical covariates.
Study Design
Among patients receiving systemic chemotherapy in the metastatic setting, outcomes were compared between ERBB2 amp+ and ERBB2 amp− disease. A prespecified subgroup analysis focused on patients with left-sided, RAS/BRAF V600E wild-type, MSI-H–undetected mCRC, who are typically considered candidates for anti-EGFR therapy. Within this subgroup, outcomes were evaluated according to first-line treatment with doublet chemotherapy plus anti-EGFR antibody or doublet chemotherapy plus bevacizumab.
Results
Of 5,545 eligible patients with mCRC who underwent tissue-based CGP, 171 (3.1%) had ERBB2 amp+ disease. The median ERBB2 copy number among ERBB2 amp+ tumours was 24 (range 5–390). Notably, 82% of ERBB2 amp+ tumours were RAS/BRAF wild-type and MSI-H not detected, highlighting that many would otherwise be considered ideal candidates for anti-EGFR therapy.
A total of 4,748 patients received systemic chemotherapy and were included in survival analyses, comprising 144 ERBB2 amp+ and 4,604 ERBB2 amp− patients. Median follow-up was similar between groups (21.7 vs. 21.5 months).
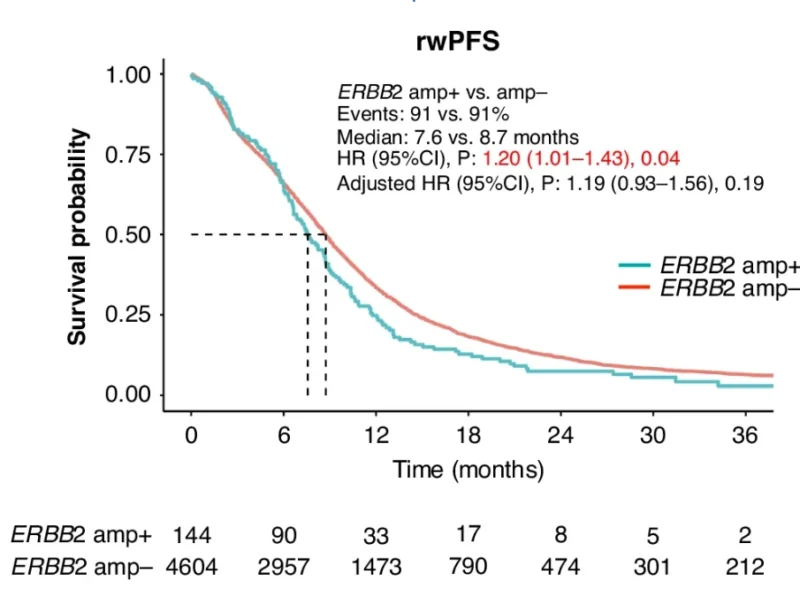
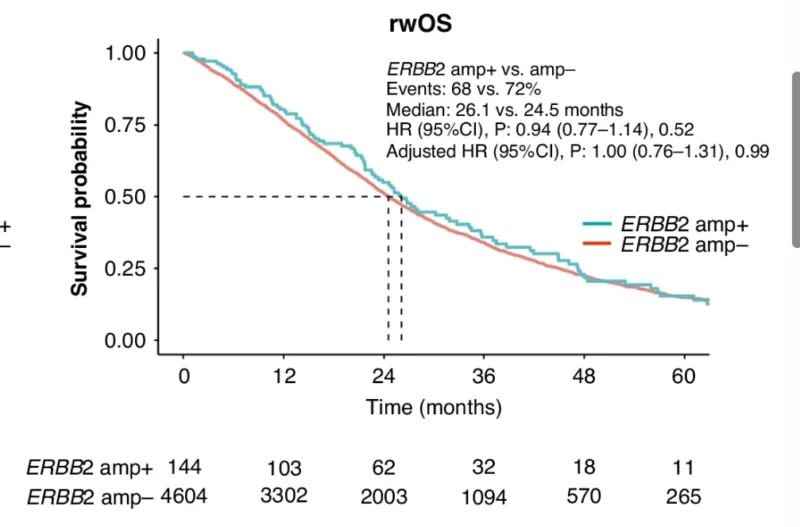
Across all first-line treatments, rwPFS was significantly shorter in ERBB2 amp+ compared with ERBB2 amp− disease (median 7.6 vs. 8.7 months; HR 1.20, 95% CI 1.01–1.43; p = 0.04). After adjustment for clinical covariates, the trend persisted but was no longer statistically significant. In contrast, rwOS did not differ between groups (median 26.1 vs. 24.5 months; HR 0.94; p = 0.52). Importantly, 27% of ERBB2 amp+ patients received anti-HER2 therapy in later lines, which may have influenced OS outcomes.
In the clinically relevant subgroup of left-sided, RAS/BRAF wild-type, MSI-H–undetected mCRC, ERBB2 amplification remained associated with inferior rwPFS. Median rwPFS was 8.6 months in ERBB2 amp+ versus 10.3 months in ERBB2 amp− patients (HR 1.47, p < 0.01; adjusted HR 1.43, p = 0.03). No significant difference in rwOS was observed (30.2 vs. 32.2 months).
When stratified by first-line biologic agent, ERBB2 amplification predicted shorter rwPFS in both treatment strategies. Among patients receiving doublet chemotherapy plus anti-EGFR antibody, median rwPFS was 8.7 months in ERBB2 amp+ versus 12.5 months in ERBB2 amp− disease (adjusted HR 2.33, p = 0.046). Similarly, in patients treated with doublet chemotherapy plus bevacizumab, median rwPFS was 8.9 vs. 10.5 months, respectively (adjusted HR 1.75, p = 0.04). A direct comparison between anti-EGFR–based and bevacizumab-based regimens within the ERBB2 amp+ group showed no significant difference in rwPFS, suggesting that neither approach overcomes the adverse prognostic impact of ERBB2 amplification in the first-line setting.
Key Findings
ERBB2 amplification was identified in 3.1% of patients with mCRC and was associated with shorter real-world PFS across standard first-line chemotherapy regimens. This effect persisted in patients with otherwise favorable molecular profiles and was observed with both anti-EGFR– and bevacizumab-based strategies. No clear difference in OS was detected, likely reflecting subsequent HER2-targeted therapies.
Conclusion
This large real-world analysis demonstrates that ERBB2 amp+ mCRC is associated with inferior rwPFS regardless of first-line biologic strategy, highlighting the limitations of current chemotherapy-based regimens in this population. These findings support ongoing efforts to evaluate HER2-targeted therapies in the first-line setting, with trials such as MOUNTAINEER-03 aiming to redefine the treatment paradigm for HER2-positive mCRC.
You can read the full article here.


