Dual immune checkpoint blockade in advanced non-small-cell lung cancer (NSCLC) has emerged as a promising approach to overcome resistance to single-agent immunotherapy. A new Lancet Oncology meta-analysis reconstructed individual patient data from six phase III trials to compare CTLA-4 plus PD-1/PD-L1 inhibition with PD-1/PD-L1 blockade alone. While overall survival outcomes were similar across the general population, the study revealed notable long-term survival benefits for patients with PD-L1–negative tumors and STK11 mutations. These findings underscore the growing role of biomarker-driven immunotherapy selection in refining treatment strategies for advanced NSCLC.
Title: Long-term overall survival with dual CTLA-4 and PD-L1 or PD-1 blockade and biomarker-based subgroup analyses in patients with advanced non-small-cell lung cancer: a systematic review and reconstructed individual patient data meta-analysis
Authors: Alessandro Di Federico, MD, Sara Stumpo, MD, Francesco Mantuano, MD, Andrea De Giglio, MD, Francesca Lo Bianco, MD, Federica Pecci, MD, Joao V Alessi, MD, Xinan Wang, PhD, Francesca Sperandi, MD, Barbara Melotti, MD, Francesco Gelsomino, MD, Ferdinandos Skoulidis, MD, Prof Marina C Garassino, MD, Prof Solange Peters, MD, Mark M Awad, MD, Prof Andrea Ardizzoni, MD, Biagio Ricciuti, MD PhD.
Published in Lancet Oncology, October 2025
Background
Immune checkpoint inhibitors (ICIs) targeting PD-1 or PD-L1 have transformed the treatment landscape of advanced non-small-cell lung cancer (NSCLC), establishing them as the standard of care for patients without actionable genomic drivers. In recent years, combinations involving PD-1/PD-L1 inhibitors and CTLA-4 blockade have demonstrated clinical activity and survival advantages over chemotherapy, particularly in patients with low PD-L1 expression. However, the benefit of adding a CTLA-4 inhibitor to PD-1 or PD-L1 blockade compared with PD-1 or PD-L1 inhibitor monotherapy remains unclear.
Moreover, identifying biomarkers that predict which subgroups of patients derive long-term benefit from dual immune checkpoint blockade is an area of active investigation. This meta-analysis sought to clarify whether combining CTLA-4 inhibition with PD-1 or PD-L1 blockade yields superior long-term overall survival (OS) compared with single-agent PD-1/PD-L1 therapy, and to define the patient populations most likely to benefit.
Methods
A systematic search of PubMed, MEDLINE, and Embase databases was performed for randomized phase III clinical trials published up to November 21, 2024. Eligible studies compared PD-1 or PD-L1 inhibitors (alone or with chemotherapy) with dual CTLA-4 and PD-1/PD-L1 blockade in previously untreated advanced NSCLC. Only trials reporting long-term survival (≥5-year follow-up) or biomarker-based subgroup analyses were included. Six randomized clinical trials met these criteria: CheckMate 227, CheckMate 9LA, POSEIDON, KEYNOTE-189, KEYNOTE-407, and IMpower150.
Individual patient data were digitally reconstructed from Kaplan–Meier survival curves using WebPlotDigitizer v5 and the IPDfromKM method. The primary endpoint was 5-year overall survival in the overall population and predefined subgroups according to PD-L1 tumor proportion score (TPS), histology, and KRAS or STK11 mutational status. KEAP1 mutations and progression-free survival (PFS) were analyzed exploratively. Statistical analysis was performed using R software with Cox proportional hazards models, and violations of proportional hazard assumptions were addressed with time-dependent models. The study followed PRISMA guidelines and was registered with PROSPERO (CRD420251081707).
Study Design and Population
A total of 2,881 patients were included: 1,282 received dual CTLA-4 and PD-1/PD-L1 blockade, and 1,599 received single PD-1/PD-L1 inhibition. Among them, 29.1% were female and 70.9% male. Median follow-up was 64.9 months. All trials enrolled treatment-naïve patients with advanced or metastatic NSCLC and an ECOG performance status of 0–1; controlled or asymptomatic brain metastases were permitted.
The included studies investigated key ICI regimens:
- Nivolumab + ipilimumab (± chemotherapy) – CheckMate 227 and 9LA
- Durvalumab + tremelimumab + chemotherapy – POSEIDON
- Pembrolizumab ± chemotherapy – KEYNOTE-189 and KEYNOTE-407
- Atezolizumab + chemotherapy – IMpower150
Genomic testing across studies utilized tissue-based or blood-based next-generation sequencing and whole-exome sequencing for KRAS, STK11, and KEAP1 mutation assessment.
Results
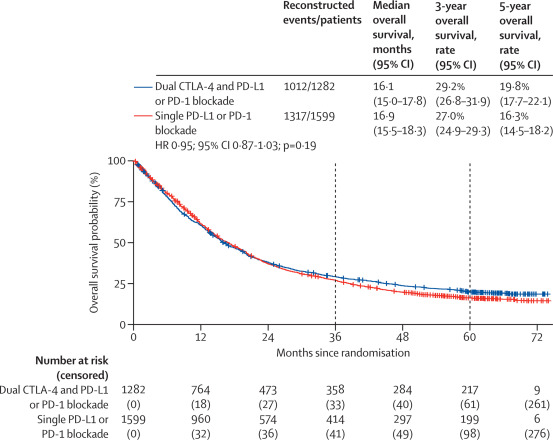
Across the overall population, median OS was similar between dual and single checkpoint blockade:
16.1 months (95% CI 15.0–17.8) vs 16.9 months (15.5–18.3), with a hazard ratio (HR) of 0.95 (95% CI 0.87–1.03; p=0.19).However, 5-year OS rates favored dual blockade: 19.8% vs 16.3%.
PD-L1 Expression
Among patients with PD-L1 TPS <1%, dual therapy significantly improved median OS:
15.5 months (13.6–18.5) vs 14.5 months (13.4–15.9); HR 0.85 (95% CI 0.74–0.98; p=0.021).
The 5-year OS rate nearly doubled — 16.6% (13.4–20.6) vs 9.3% (7.0–12.3) with single-agent PD-1/PD-L1 inhibition.
Among patients with PD-L1 TPS ≥1%, outcomes were comparable: 16.5 vs 18.1 months (HR 0.97; p=0.60). Time-dependent analysis revealed a delayed survival advantage with dual blockade after 36 months.
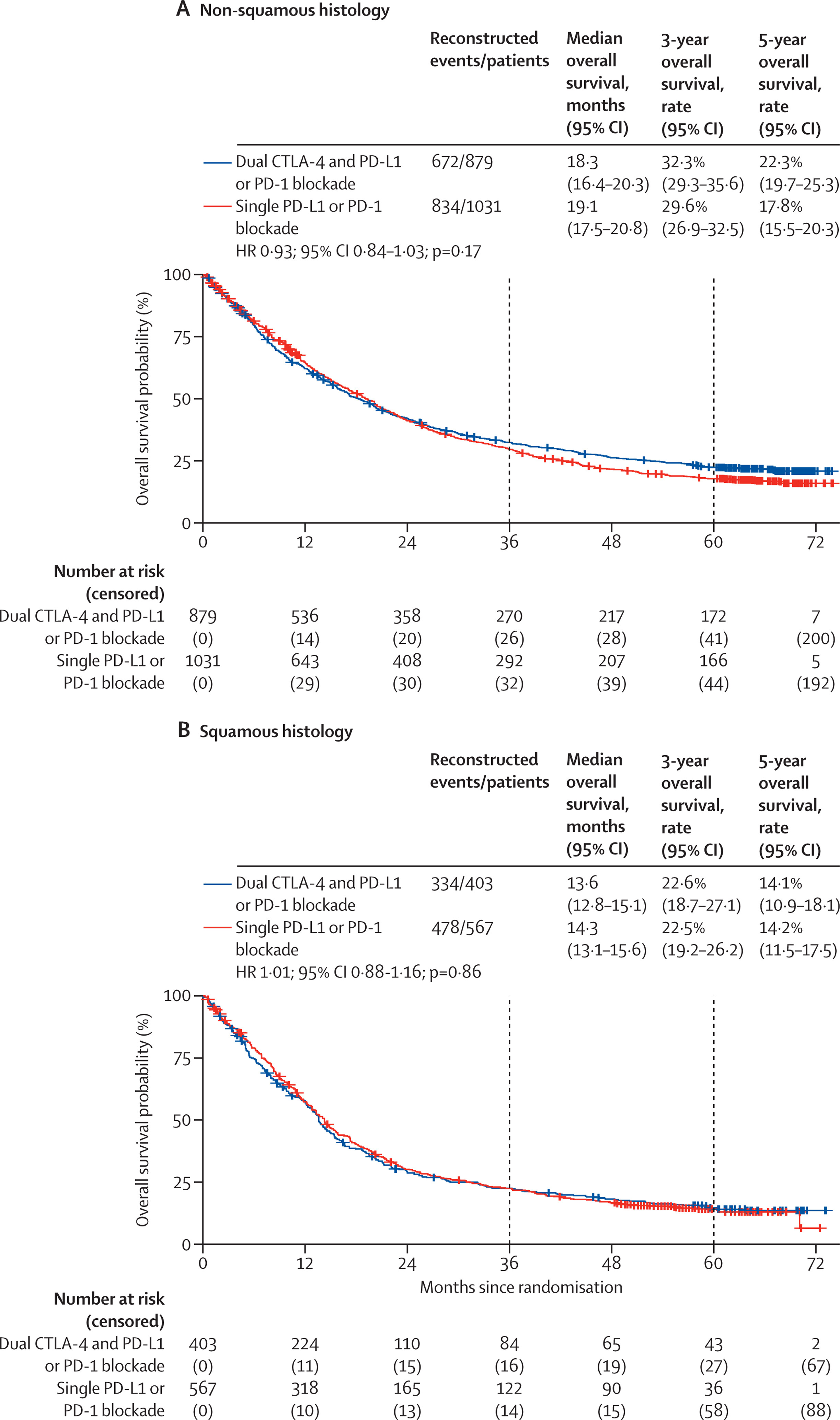
Tumor Histology
No significant OS difference was observed between non-squamous or squamous histologies for dual vs single blockade. However, time-dependent analyses again indicated a trend favoring dual blockade over longer follow-up.
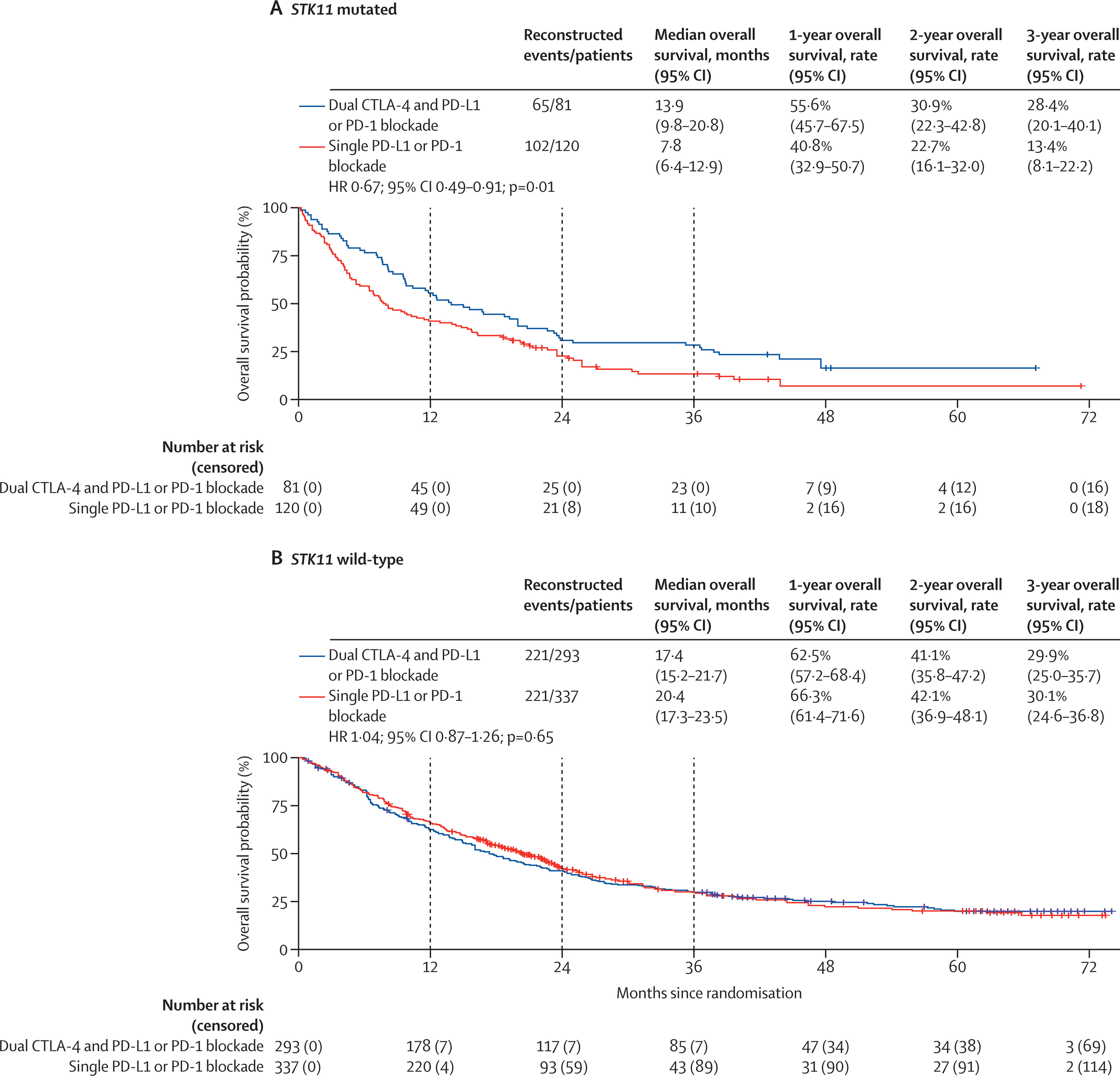
Molecular Subgroups
Among STK11-mutated NSCLC, dual checkpoint blockade achieved a pronounced OS benefit:
13.9 months (9.8–20.8) vs 7.8 months (6.4–12.9); HR 0.67 (95% CI 0.49–0.91; p=0.012).
In contrast, patients with STK11 wild-type tumors had no significant difference in survival outcomes.
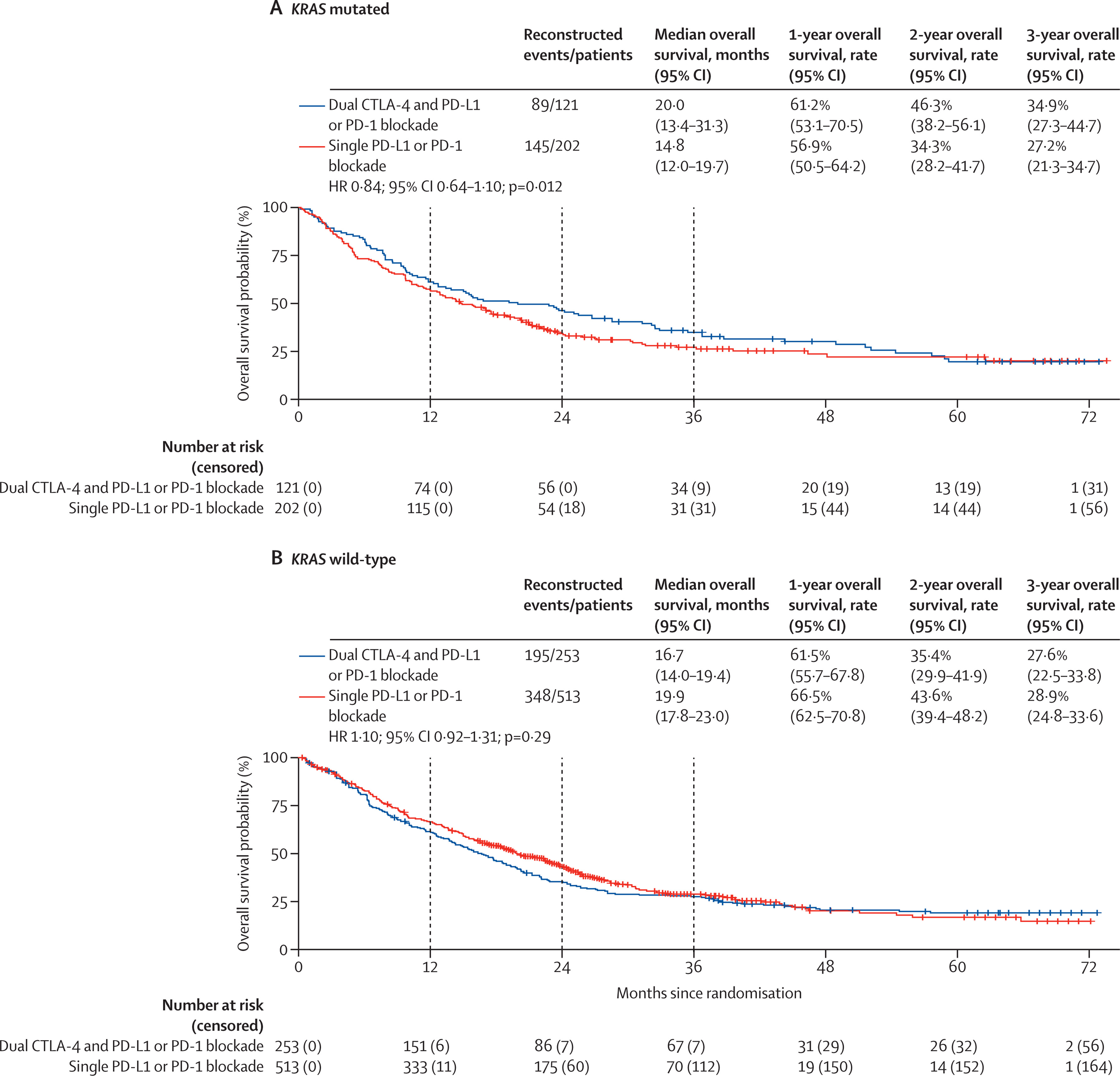
For KRAS-mutated NSCLC (n=323), median OS was not significantly different between groups. The same was true for KRAS wild-type tumors. Exploratory analysis of KEAP1-mutated tumors showed a numerical but nonsignificant improvement with dual blockade.
Progression-Free Survival
Median PFS did not significantly differ between treatment arms, reinforcing that the long-term benefit of dual checkpoint inhibition is primarily reflected in OS beyond 3 years.
Key Findings
- Dual CTLA-4 and PD-1/PD-L1 blockade provided similar median OS to single-agent PD-1/PD-L1 inhibitors in the overall population but conferred higher 5-year survival rates.
- Patients with PD-L1 TPS <1% achieved nearly double 5-year OS with dual blockade (16.6% vs 9.3%).
- STK11-mutated tumors derived a clinically meaningful OS improvement (HR 0.67; p=0.012).
- No significant advantage was observed by KRAS status or tumor histology.
- Time-dependent hazard modeling demonstrated a late-emerging survival benefit with dual therapy, emphasizing the need for long-term follow-up in immunotherapy trials.
Key Takeaway Messages
Dual checkpoint inhibition should not be routinely used in all patients with advanced NSCLC but may offer substantial benefit in specific subgroups—particularly those with PD-L1–negative tumors and STK11 mutations, both associated with immune resistance to single-agent PD-1/PD-L1 blockade.
The findings also reinforce that traditional proportional hazard models may underestimate delayed survival benefits typical of immunotherapy, highlighting the value of extended follow-up and time-dependent analyses.
Ongoing trials, including TRITON (NCT06008093), are expected to validate these results and refine patient selection for combination immunotherapy.
Conclusion
This systematic review and reconstructed individual patient data meta-analysis demonstrate that dual immune checkpoint blockade with CTLA-4 and PD-1/PD-L1 inhibitors achieves comparable overall survival to monotherapy in unselected patients with advanced NSCLC but yields notable long-term advantages in PD-L1–negative and STK11-mutated subgroups.
These data support a biomarker-driven approach to immunotherapy selection, reserving dual blockade for patients with features predictive of low response to PD-1/PD-L1 inhibitors alone. While toxicity and cost considerations remain important, this study provides strong rationale for personalized integration of CTLA-4 inhibition in specific NSCLC populations, paving the way for more targeted, durable immunotherapy strategies.
You can read the full article here.