The CATNON trial is the only randomized phase III study to separately test temozolomide given during radiotherapy versus after radiotherapy in patients with newly diagnosed 1p/19q non–co-deleted anaplastic glioma, and this final report provides the most mature survival data in the modern molecular era by focusing on the subgroup with IDH-mutant tumors after more than a decade of follow-up. By integrating long-term clinical outcomes with deep methylation and genomic profiling, the analysis clarifies which component of temozolomide treatment truly drives benefit and whether key molecular risk features alter that effect—questions that directly shape today’s standard approach to grade 3 astrocytoma.
Titile: Concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): final and exploratory analyses of a randomised, open-label, phase 3 trial
Authors: Prof Martin J van den Bent, MD, Santoesha A Ghisai, MSc, Prof Wolfgang Wick, MD, Prof Marc Sanson, MD, Alba Ariela Brandes, MD, Prof Paul M Clement, MD, Sara C Erridge, MD, Prof Michael A Vogelbaum, MD, Prof Anna K Nowak, MD, Prof Jean-François Baurain, MD, Prof Warren P Mason, MD, Helen Wheeler, MD, Prof Emeline Tabouret, MD, Sanjeev Gill, MD, Matthew Griffin, MD, Walter Taal, MD, Prof Roberta Rudà, MD, Prof Michael Weller, MD, Catherine McBain, MD, Jaap C Reijneveld, MD, Roelien H Enting, MD, Sébastien Tran, MD,
Thierry Lesimple, MD, Martin Kocher, MD, Anja Gijtenbeek, MD, Elizabeth Lim, MD, Prof Ulrich Herrlinger, MD, Prof Peter Hau, MD, Frederic Dhermain, MD, Kenneth Aldape, MDPro, f Robert B Jenkins, MD, Hendrikus Jan Dubbink, PhD, Prof Johan M Kros, MD, Prof Pieter Wesseling, MD, Youri Hoogstrate, PhD, Sarah Nuyens, Vassilis Golfinopoulos, MD, C Mircea S Tesileanu, MD, Thierry Gorlia, PhD, Prof Pim French, PhD, Brigitta G Baumert, MD
Published in Lancet Oncology, January 2026
Background
The CATNON trial was designed to answer a practical question in grade 3 diffuse glioma without 1p/19q co-deletion: does adding temozolomide to radiotherapy improve survival, and if so, is the benefit driven by temozolomide given during radiotherapy (concurrent) or after radiotherapy (adjuvant)?
Since the trial opened, the biology-based classification of diffuse glioma has changed substantially, with isocitrate dehydrogenase (IDH) status becoming central to diagnosis and prognosis. This final report focuses on long-term outcomes, particularly in the subgroup with IDH-mutant tumors, where survival is long enough that mature overall survival (OS) data require extended follow-up.
Methods
This was a randomized, open-label, phase 3 study conducted across 137 institutions in Australia, Europe, and North America. Eligible patients were ≥18 years with newly diagnosed 1p/19q non–co-deleted anaplastic glioma and WHO performance status 0–2, with adequate organ function. Central pathology confirmation and assessment of 1p/19q status were required (with specific provisions for approved local testing), and MGMT promoter methylation by qPCR was used for stratification during the study. An important protocol amendment (June 27, 2011) incorporated IDH mutational status into the analyses, reflecting its emerging role as a disease-defining biomarker.
The primary endpoint was overall survival (OS), adjusted by stratification factors, analyzed in the intention-to-treat population. Secondary endpoints included progression-free survival (PFS) and other clinical/functional outcomes (reported elsewhere). Safety had been published previously; this final report does not present new safety signals.
Study Design
The trial used a 2×2 factorial design, randomizing patients 1:1:1:1 to four arms:
- Radiotherapy (RT) alone: 59.4 Gy in 33 fractions
- RT + concurrent temozolomide: oral temozolomide 75 mg/m² daily during RT
- RT + adjuvant temozolomide: 12 cycles of temozolomide (150–200 mg/m², days 1–5 every 4 weeks) starting ~4 weeks after RT
- RT + concurrent + adjuvant temozolomide
Randomization was stratified by institution, performance status, age (>50 vs ≤50), 1p loss of heterozygosity, oligodendroglial elements on microscopy, and MGMT promoter methylation status (methylated vs unmethylated vs undetermined/invalid).
Beyond the clinical comparison, the investigators performed deep molecular profiling in the IDH-mutant astrocytoma subgroup, including next-generation sequencing, DNA methylation profiling (Illumina EPIC), copy-number variation (CNV) extraction from methylation arrays, and methylation-based tumor classification using two platforms. They also evaluated methylation-derived grading scores (CGC and GMS) and explored whether molecular features were prognostic and/or predictive of benefit from temozolomide.
Results
Enrollment and follow-up. Between Dec 4, 2007 and Sept 11, 2015, 1407 patients were registered and 751 were randomized. The database lock for the present analysis was Oct 1, 2024, with a median follow-up of 10.8 years for PFS and 10.9 years for OS. At lock, there were 573 PFS events (76%) and 499 OS events (66%).
IDH-defined subgroups. Adequate tumor material for IDH testing was available for 671/751 (89%) randomized patients. An IDH mutation was identified in 455 tumors; 11 of these had 1p/19q co-deletion, leaving 444 with IDH-mutant, 1p/19q-intact disease (reported as astrocytoma IDH-mutant under modern classifications). 216 tumors were IDH wild-type. Survival differed dramatically by IDH status: median OS was 1.7 years (95% CI 1.4–1.9) for IDH wild-type versus 8.5 years (7.1–9.9) for astrocytoma IDH-mutant (HR 0.18, 95% CI 0.15–0.22, comparing IDH-mutant vs IDH wild-type).
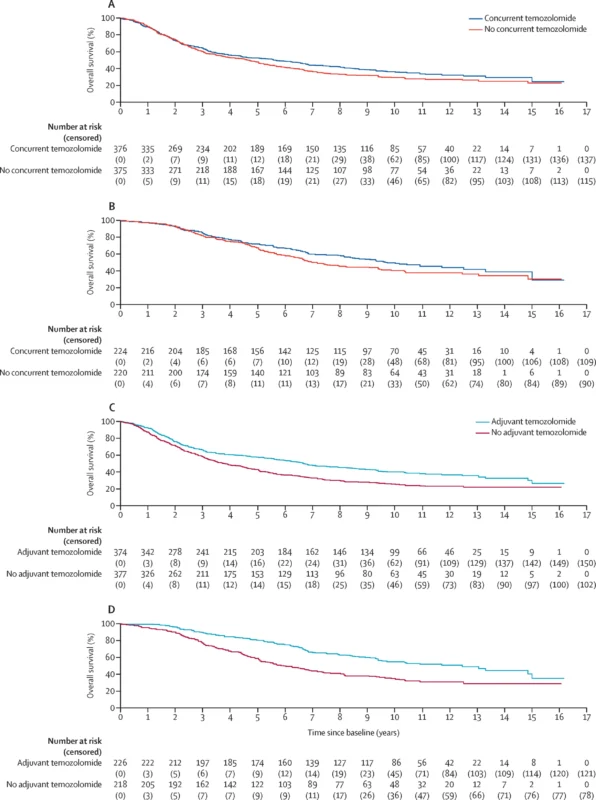
Primary treatment effects (intention-to-treat).
- Concurrent temozolomide: No OS improvement versus no concurrent temozolomide (HR 0.91, 95% CI 0.76–1.08).
- Adjuvant temozolomide: Clear OS benefit versus no adjuvant temozolomide (HR 0.65, 95% CI 0.54–0.77).
A secondary model replacing MGMT stratification with IDH status showed the same pattern: concurrent remained non-significant (HR 0.88, 0.73–1.06), while adjuvant remained beneficial (HR 0.71, 0.59–0.86).
IDH-mutant subgroup (key clinical signal). In 444 patients with IDH-mutant tumors:
- Concurrent temozolomide showed no statistically significant OS benefit (HR 0.81, 95% CI 0.63–1.04).
- Median OS was 9.7 years (8.2–12.5) with concurrent temozolomide vs 7.2 years (6.2–9.4) without.
Adjuvant temozolomide produced a substantial OS improvement (HR 0.54, 95% CI 0.42–0.69). Median OS was 12.5 years (9.4–15.0) with adjuvant temozolomide vs 6.0 years (5.1–7.2) without.
IDH wild-type subgroup. In IDH wild-type tumors, neither concurrent (HR 1.04, 0.78–1.38) nor adjuvant temozolomide (HR 1.02, 0.77–1.36) improved OS, supporting the conclusion that benefit is restricted to IDH-mutant disease in this population.
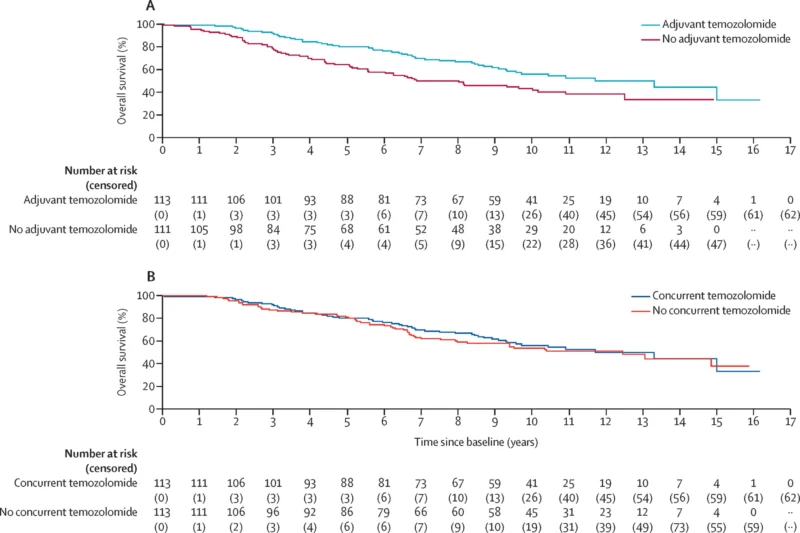
Exploratory “disentangling” analysis in IDH-mutant astrocytoma. When the investigators looked within the IDH-mutant group at sequencing of concurrent and adjuvant therapy, the survival advantage consistently aligned with adjuvant temozolomide. Among those receiving adjuvant therapy, adding concurrent temozolomide did not improve OS (HR 0.92, 0.63–1.36). This strengthens the interpretation that the long-term survival signal is driven by the post-radiotherapy temozolomide course.
Therapy after progression. Post-progression treatment was common: among 573 patients with progression, 455 (79%) received further therapy. Chemotherapy was given in 427 (75%), including temozolomide in 313 (55%), PCV in 109 (19%), lomustine in 188 (33%), and bevacizumab in 167 (29%). Notably, among patients initially treated with RT alone who later progressed (n=163), 129 (79%) received chemotherapy at progression and 119 (73%) received temozolomide—yet this did not “catch up” to the benefit seen with upfront adjuvant temozolomide in IDH-mutant disease.
Molecular prognostic factors (IDH-mutant astrocytoma). Several alterations were associated with worse outcomes, including homozygous deletion of CDKN2A/CDKN2B (median OS 3.0 years vs 9.6 years without; HR 3.58), PDGFRA amplification (median OS 3.1 years vs 9.3 years; HR 3.51), PTEN loss of heterozygosity (HR 2.38), and high total CNV load (HR 1.60). Importantly, none of these features was predictive for who benefits from temozolomide: interaction testing did not identify a subgroup in which temozolomide benefit disappeared.
MGMT promoter methylation (predictive value). MGMT status, assessed by qPCR and a methylation-array algorithm, was poorly correlated and—crucially—was not prognostic or predictive for temozolomide benefit in IDH-mutant astrocytoma in these analyses.
Key findings
- In the full randomized cohort, adjuvant temozolomide improved OS (HR 0.65), while concurrent temozolomide did not (HR 0.91).
- The benefit is restricted to IDH-mutant tumors: in IDH-mutant disease, adjuvant temozolomide improved median OS to 12.5 years versus 6.0 years without adjuvant therapy (HR 0.54).
- In IDH wild-type tumors, temozolomide (concurrent or adjuvant) showed no OS benefit.
Multiple molecular features were strongly prognostic (e.g., CDKN2A/B homozygous deletion, PDGFRA amplification), but none predicted temozolomide benefit—supporting treatment across molecular risk strata within IDH-mutant astrocytoma.
Conclusion
This mature, long-term analysis clarifies the practical message of CATNON in modern molecular terms: for aggressive IDH-mutant astrocytoma, the survival benefit of adding temozolomide to radiotherapy is delivered primarily—if not entirely—through 12 cycles of adjuvant temozolomide, with no convincing evidence that concurrent temozolomide adds meaningful overall survival benefit.
The dataset also reinforces that IDH status is the dominant determinant of outcome, while additional genomic and methylation-derived markers refine prognosis without identifying a subgroup that should be spared adjuvant temozolomide. In a disease where median survival can extend beyond a decade, these results provide a stable foundation for current standards and a benchmark against which emerging strategies—particularly IDH inhibitors in higher-grade disease—will need to compete.


