Astrocytoma is a complex and biologically diverse group of primary brain tumors that arise from astrocytes—glial cells critical for neuronal support and homeostasis in the central nervous system. This article provides an in-depth overview of astrocytoma, from its classification under the latest 2021 WHO CNS tumor criteria to its clinical presentation, diagnostic approach, and treatment strategies tailored by molecular subtype. With the growing relevance of personalized medicine, we also explore cutting-edge research and clinical trials active in 2025, including IDH-targeted therapies, CDK9 inhibitors, immunotherapy, and Tumor Treating Fields. Whether you’re a clinician, researcher, or an informed patient, this resource offers a comprehensive look at the evolving landscape of astrocytoma care.
What Is Astrocytoma?
Astrocytoma is a type of primary central nervous system (CNS) tumor that arises from astrocytes, a subtype of glial cells that play essential roles in maintaining the blood-brain barrier, providing nutrients to neurons, regulating synaptic transmission, and supporting brain repair after injury. Astrocytomas are classified under the broader category of diffuse gliomas, which encompass a spectrum of infiltrative glial neoplasms characterized by varying degrees of cellular atypia, mitotic activity, microvascular proliferation, and necrosis.
Astrocytomas can affect patients of any age, but their biological behavior and clinical presentation differ by age group. Pilocytic astrocytomas (WHO Grade I), for instance, are more commonly seen in children and are typically circumscribed and non-infiltrative. In contrast, diffuse astrocytomas (WHO Grades II–IV) are most common in adults and exhibit progressive, infiltrative growth into surrounding brain parenchyma.
Astrocytoma Types
Astrocytomas are primary brain tumors that originate from astrocytes, a type of glial cell in the central nervous system. The classification of astrocytomas has evolved significantly, especially with the incorporation of molecular features in the 2021 World Health Organization (WHO) Classification of CNS Tumors. Tumors are now categorized based not only on histological characteristics but also on genetic and molecular markers that influence prognosis and treatment.Astrocytomas are classified under the broader category of diffuse gliomas and are subdivided as follows:
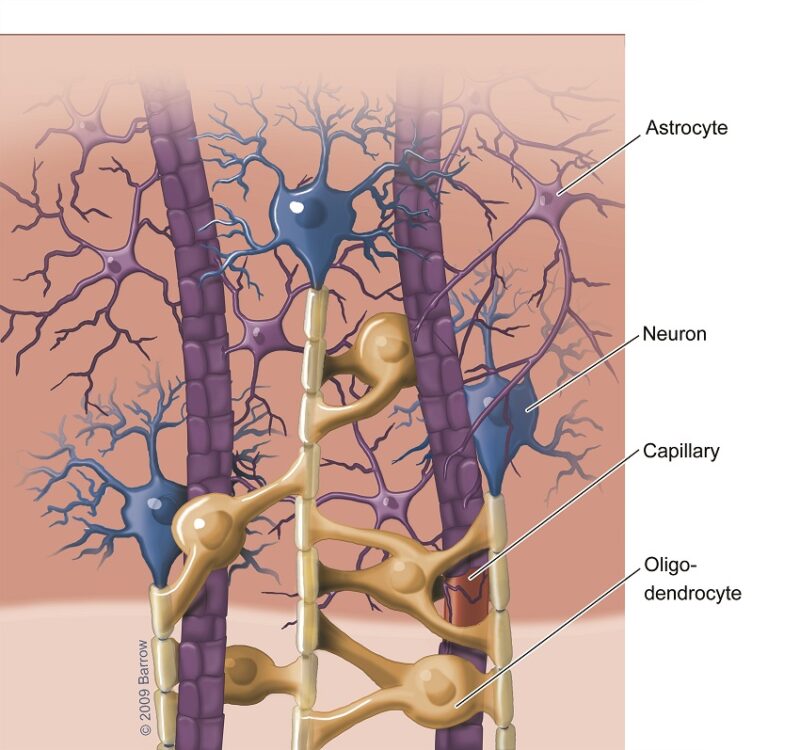
Diffuse Astrocytoma, IDH-mutant, WHO Grade 2
These tumors are characterized by infiltrative growth, relatively low cellularity, and nuclear atypia but no mitotic activity, necrosis, or microvascular proliferation. The presence of an IDH1 or IDH2 mutation is essential for this diagnosis. These tumors typically have a more favorable prognosis compared to IDH-wildtype gliomas and are commonly seen in younger adults (Louis et al., 2021).
Anaplastic Astrocytoma, IDH-mutant, WHO Grade 3
This is a more aggressive variant that shows increased mitotic activity. Like Grade 2 astrocytomas, it must harbor an IDH mutation. These tumors have a worse prognosis than Grade 2 astrocytomas but better outcomes than glioblastomas. The presence or absence of CDKN2A/B homozygous deletion may further affect prognosis (Wesseling et al., 2021).
Astrocytoma, IDH-mutant, Grade 4
This diagnosis is made in IDH-mutant tumors that also show microvascular proliferation and/or necrosis, hallmark features of high-grade malignancy. This is the most aggressive form of IDH-mutant astrocytomas and requires intensive treatment. It replaces the older term “secondary glioblastoma” (Louis et al., 2021).
Glioblastoma, IDH-wildtype
Although not labeled as an “astrocytoma” in modern classification, glioblastoma often arises from astrocytic lineage and is included in this context. It is characterized by rapid growth, necrosis, and microvascular proliferation. It usually affects older adults and has a very poor prognosis. Molecular features such as TERT promoter mutation, EGFR amplification, and chromosome 7 gain/10 loss are key diagnostic markers (Weller et al., 2021).

Read More About Glioblastoma on Oncodaily
What Causes Astrocytoma?
The exact causes of astrocytoma, a tumor arising from astrocytes (star-shaped glial cells in the brain and spinal cord), remain largely unclear. However, several genetic, environmental, and biological factors are known to contribute to its development.
Genetic mutations are among the most prominent contributors. Mutations in genes such as TP53, IDH1/2, and ATRX are frequently found in low- and high-grade astrocytomas. In glioblastomas (grade 4 astrocytomas), EGFR amplification and PTEN loss are common genetic alterations (Louis et al., 2021). These mutations lead to abnormal cell growth and resistance to cell death. Inherited genetic syndromes like Li-Fraumeni syndrome, Neurofibromatosis type 1, and Turcot syndrome increase the risk of developing astrocytomas, particularly in children and young adults (Weller et al., 2021).
Environmental exposures have a less defined role. Ionizing radiation, particularly therapeutic cranial radiation, is one of the few environmental risk factors consistently linked to increased astrocytoma risk (Preston et al., 2007). The evidence for other exposures, such as to chemicals or cell phone radiation, remains inconclusive. Age and gender also influence risk. Astrocytomas can occur at any age but are most common in adults between 30 and 60 years. High-grade astrocytomas, such as glioblastomas, occur more frequently in men than in women. Immune system dysfunction and chronic inflammation in the central nervous system are being explored as potential contributors, although direct causal relationships are still under investigation.
Symptoms of Astrocytoma
The symptoms of astrocytoma vary significantly depending on the tumor’s location, grade, and rate of growth. Early-stage, low-grade astrocytomas may cause subtle symptoms, while high-grade glioblastomas often present with rapidly progressing neurological deficits. Common symptoms of astrocytoma include:
- Headaches, often worse in the morning or with changes in position, due to increased intracranial pressure.
- Seizures, particularly in low-grade astrocytomas, can be a presenting symptom in up to 50–80% of patients.
- Cognitive or personality changes, including memory loss, confusion, or decreased attention span.
- Motor weakness or sensory disturbances, typically due to involvement of motor or sensory cortex.
- Speech difficulties, especially with tumors in the dominant hemisphere’s temporal or frontal lobes.
- Visual disturbances, including blurred vision or visual field deficits, may occur with occipital or optic pathway involvement.
- Nausea and vomiting, often due to increased intracranial pressure.
In pediatric patients, astrocytomas (especially pilocytic astrocytomas) often arise in the posterior fossa, leading to symptoms like gait disturbances, dizziness, and hydrocephalus from obstructed cerebrospinal fluid flow.
How Is Astrocytoma Diagnosed?
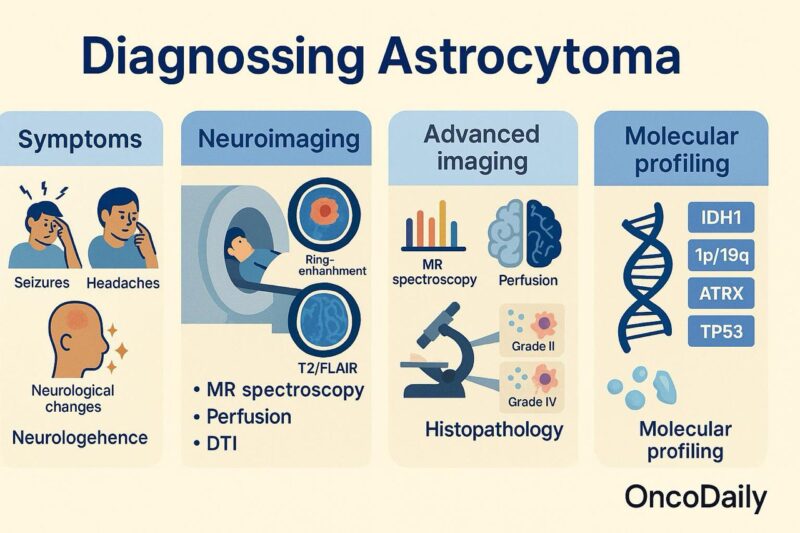
Diagnosing astrocytoma involves a comprehensive approach integrating clinical evaluation, neuroimaging, histopathological analysis, and molecular profiling. Symptoms such as seizures, headaches, and neurological deficits often prompt imaging studies, which are central to the initial detection and assessment of the tumor.
Magnetic Resonance Imaging (MRI) is the preferred imaging modality due to its superior soft-tissue contrast. T1-weighted images with contrast help delineate the tumor boundaries, while T2 and FLAIR sequences assess edema and infiltration. Low-grade astrocytomas (WHO grade II) usually appear as non-enhancing lesions, whereas high-grade astrocytomas (grades III and IV, including glioblastoma) show ring-enhancement, necrosis, and mass effect (Louis et al., 2021). Advanced imaging techniques such as MR spectroscopy, perfusion MRI, and diffusion tensor imaging can provide further insight into tumor metabolism, vascularity, and invasion patterns, aiding in differentiating tumor types and guiding surgical planning (Weller et al., 2021).
A definitive diagnosis requires histopathological examination, obtained through biopsy or surgical resection. Histological grading is based on features like cellularity, mitotic activity, microvascular proliferation, and necrosis.
Molecular diagnostics have become essential for classification and prognosis. Important markers include:
- IDH mutation status (IDH1/IDH2), which distinguishes astrocytomas into IDH-mutant (better prognosis) and IDH-wildtype (often more aggressive, glioblastoma-like).
- ATRX loss and TP53 mutations, typically seen in IDH-mutant astrocytomas.
- MGMT promoter methylation, which predicts response to temozolomide.
- 1p/19q codeletion, the absence of which supports the diagnosis of astrocytoma over oligodendroglioma (Louis et al., 2021).
The 2021 WHO CNS tumor classification emphasizes a layered diagnostic approach, integrating histological and molecular features to provide a more precise and clinically relevant tumor classification.
Prognosis of Astrocytoma
The prognosis of astrocytoma varies significantly depending on the tumor’s WHO grade, molecular profile, patient age, extent of resection, and response to therapy. Astrocytomas are categorized into grades 1 through 4, with lower grades (I and II) generally associated with a more favorable prognosis, and higher grades (III and IV) linked to poor outcomes due to aggressive growth and infiltrative behavior.
Grade I (Pilocytic astrocytoma) usually occurs in children and young adults and is considered a benign tumor. When completely resected, it is often curable, with 10-year survival rates exceeding 90% (Louis et al., 2021).
Grade II (Diffuse astrocytoma) tends to affect younger adults and has a more indolent course. However, it has a high risk of progressing to a higher-grade glioma over time. The median survival is typically around 5 to 10 years, especially in patients with IDH-mutant and 1p/19q non-codeleted tumors (Weller et al., 2021).
Grade III (Anaplastic astrocytoma) is more aggressive. Patients with IDH-mutant anaplastic astrocytomas have better outcomes, with a median survival of 6 to 10 years, compared to poorer outcomes for IDH-wildtype cases, which behave more like glioblastoma (Louis et al., 2021).
Grade IV (Glioblastoma) represents the most aggressive form of astrocytoma and has a median overall survival of 12 to 15 months with standard therapy. The prognosis worsens without favorable markers like MGMT promoter methylation or IDH mutation (Hegi et al., 2005; Stupp et al., 2005).
Important molecular features that impact prognosis include:
- IDH mutation: Associated with significantly improved survival across grades II and III.
- ATRX loss and TP53 mutation: Common in astrocytomas and influence tumor behavior.
- MGMT promoter methylation: Predicts better response to alkylating agents like temozolomide in high-grade tumors.
Extent of surgical resection also critically impacts prognosis. Gross total resection is associated with longer survival compared to subtotal resection or biopsy alone (Weller et al., 2021).
Treatment of Astrocytoma
The treatment of astrocytoma is determined by tumor grade, location, molecular profile, and patient-specific factors such as age and performance status. The World Health Organization (WHO) classifies astrocytomas into four grades (I–IV), and treatment strategies vary significantly across this spectrum.
Grade I (Pilocytic Astrocytoma): This type typically occurs in children and young adults. Surgery is the mainstay of treatment. Gross total resection is often curative, and no additional therapy is needed if complete resection is achieved. If subtotal resection is performed or recurrence occurs, options include repeat surgery or radiotherapy (Louis et al., 2021).
Grade II (Diffuse Astrocytoma, IDH-mutant): These low-grade tumors are often slow-growing but infiltrative. The optimal treatment involves maximal safe surgical resection. Observation may follow if the patient is young with a small tumor and complete resection. However, for larger, symptomatic, or incompletely resected tumors, adjuvant therapy with radiotherapy and/or chemotherapy (e.g., temozolomide or PCV regimen) is considered, especially in higher-risk patients (Weller et al., 2021).

Read More About Radiotherapy for Brain Tumors on Oncodaily
Grade III (Anaplastic Astrocytoma, IDH-mutant): This intermediate-grade tumor typically requires a multimodal approach. After surgical resection, adjuvant radiotherapy followed by chemotherapy with temozolomide is standard. IDH mutation status is important as it influences both prognosis and response to therapy. Patients with IDH-mutant tumors have better survival outcomes (Weller et al., 2021).
Grade IV (Glioblastoma or Astrocytoma, IDH-wildtype): Previously known as glioblastoma, this is the most aggressive form of astrocytoma. Treatment includes maximal safe resection, followed by concurrent chemoradiotherapy (60 Gy radiation with daily temozolomide) and adjuvant temozolomide for 6 cycles, known as the Stupp protocol. The addition of tumor-treating fields (TTFields) may also be offered to prolong progression-free survival (Stupp et al., 2005; Weller et al., 2021).
Molecular Features and Personalized Therapy: Molecular classification—especially IDH mutation, MGMT promoter methylation, and 1p/19q codeletion—guides treatment. IDH-mutant tumors respond better to chemotherapy, and MGMT methylation predicts benefit from temozolomide (Hegi et al., 2005). Trials are underway investigating immunotherapy, targeted inhibitors (e.g., IDH inhibitors), and vaccine-based approaches, particularly for higher-grade astrocytomas.
Read More About Immunotherapy for Brain Cancer on Oncodaily
New and Ongoing Clinical Trials in 2025 for Astrocytoma 2025
Astrocytoma, a primary brain tumor originating from astrocytes, encompasses a wide range of malignancies from low-grade (WHO grade 2) to high-grade (anaplastic and glioblastoma). With the integration of molecular diagnostics into the WHO CNS5 classification, clinical trials have increasingly focused on IDH mutation status, 1p/19q codeletion, MGMT methylation, and other biomarkers. The following are key ongoing and recently completed trials in astrocytoma treatment.
Vorasidenib in IDH1/2-Mutant Grade 2 Astrocytoma
Vorasidenib, a brain-penetrant inhibitor of mutant isocitrate dehydrogenase 1 and 2 (IDH1/2), was evaluated in a randomized, placebo-controlled Phase 3 trial known as INDIGO. The trial included patients with IDH-mutant, non-enhancing grade 2 gliomas—including astrocytomas—who had undergone surgery but had not yet received radiation or chemotherapy. The study found that vorasidenib significantly prolonged progression-free survival and delayed the need for more aggressive treatments (Mellinghoff et al., 2023), leading to FDA approval in 2024. This is the first targeted therapy approved specifically for low-grade diffuse astrocytoma with IDH mutations.
Zotiraciclib with Temozolomide in Recurrent High-Grade Gliomas
Zotiraciclib (TG02) is a selective cyclin-dependent kinase 9 (CDK9) inhibitor that has shown activity in high-grade astrocytomas. The ongoing Phase 1b/2 trial combines zotiraciclib with dose-dense temozolomide in patients with recurrent astrocytoma or glioblastoma. In a preliminary report, Raizer et al. (2021) noted manageable toxicity and encouraging signals of clinical benefit. Given the frequent MYC pathway dysregulation in astrocytomas, CDK9 inhibition is a rational strategy to suppress survival-related transcriptional programs in these tumors.
Evofosfamide in Hypoxic High-Grade Gliomas
Evofosfamide (TH-302) is a hypoxia-activated prodrug of bromo-isophosphoramide mustard, designed to become cytotoxic under low oxygen tension—a hallmark of high-grade astrocytomas. In a Phase 2 trial involving recurrent glioblastoma and anaplastic astrocytoma, evofosfamide demonstrated central nervous system penetration and acceptable safety (Jain et al., 2020). The study aims to validate whether exploiting tumor hypoxia can improve outcomes in patients with poor-prognosis astrocytomas.
Tumor Treating Fields in Grade 3 Gliomas
Tumor Treating Fields (TTFields) are low-intensity, alternating electric fields applied non-invasively via transducer arrays. While currently standard in glioblastoma, they are under active investigation in grade 3 astrocytomas. TTFields interfere with mitotic spindle formation and are hypothesized to be more effective when used early in disease. Stupp et al. (2017) demonstrated a survival advantage with TTFields in glioblastoma, and ongoing trials such as NCT04492168 are expanding their use to anaplastic astrocytomas.
Neoadjuvant Nivolumab Prior to Surgery in Grade 3 Gliomas
A new frontier in astrocytoma management is neoadjuvant immunotherapy—administering immune checkpoint inhibitors like nivolumab before surgical resection. This strategy, supported by translational immunology data (Schalper et al., 2020), aims to enhance immune priming and post-surgical tumor control. The NCT04201873 trial includes patients with IDH-wildtype or IDH-mutant grade 3 gliomas (astrocytomas) and evaluates safety, feasibility, and immune responses.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
FAQ
What is astrocytoma?
Astrocytoma is a type of brain tumor that develops from astrocytes—star-shaped glial cells in the brain and spinal cord. It ranges from low-grade (slow-growing) to high-grade (aggressive) forms.
What causes astrocytoma?
The exact cause is unknown, but common factors include genetic mutations (e.g., IDH1/2, TP53), inherited syndromes (like Li-Fraumeni), and prior exposure to cranial radiation.
What are the symptoms of astrocytoma?
Symptoms vary by tumor location and grade and may include headaches, seizures, memory loss, speech difficulties, visual changes, and motor weakness.
How is astrocytoma diagnosed?
Diagnosis involves MRI imaging, followed by biopsy or surgical resection for histopathological and molecular analysis, including IDH mutation and MGMT status.
What are the types of astrocytoma?
Types include diffuse astrocytoma (Grade 2), anaplastic astrocytoma (Grade 3), and astrocytoma Grade 4 (formerly part of glioblastoma), classified based on molecular and histological features.
How is astrocytoma treated?
Treatment depends on tumor grade and may include surgery, radiation, chemotherapy (e.g., temozolomide), and newer options like Tumor Treating Fields or IDH inhibitors.
What is the prognosis for astrocytoma?
Prognosis varies widely. Low-grade tumors can have 10-year survival, while high-grade forms like glioblastoma have median survival of 12–15 months with standard treatment.
What are the 2025 advances in astrocytoma treatment?
Recent advances include FDA approval of vorasidenib for IDH-mutant tumors, trials of zotiraciclib (CDK9 inhibitor), and neoadjuvant immunotherapy with nivolumab.
Can astrocytoma come back after treatment?
Yes. Recurrence is common, especially in higher-grade astrocytomas. Follow-up care includes regular MRI scans and may require repeat treatment or clinical trial enrollment.
Is astrocytoma the same as glioblastoma?
Not exactly. Glioblastoma is a WHO Grade 4 IDH-wildtype tumor, often considered the most aggressive form of astrocytoma. However, classification now separates them based on molecular features.


