The CAPItello-290 randomized, global phase III trial was designed to evaluate whether adding the pan-AKT inhibitor capivasertib to first-line paclitaxel could improve clinical outcomes in patients with previously untreated metastatic triple-negative breast cancer (TNBC). This study was conducted in response to strong biological and clinical rationale, including frequent activation of the PI3K/AKT/PTEN signaling pathway in TNBC and encouraging survival signals observed in the earlier phase II PAKT trial. CAPItello-290 specifically aimed to assess overall survival in both the overall population and in tumors harboring PIK3CA, AKT1, or PTEN alterations, while also characterizing progression-free survival, response rates, and safety in a large, global cohort.
Title: Capivasertib plus paclitaxel as first-line treatment for metastatic triple-negative breast cancer: Results from the randomised, global phase III CAPItello-290 trial
Authors: P.S. Schmid, H.L. McArthur, J. Cortés, B. Xu, F. Cardos, M. Casalnuovo, U. Demirci, R. Freitas-Junior, J. Ghosh, R. Hegg, H. Iwata, I. Karnaukhov, Y.L. Chuken, M. Nechaeva, M.E. Robson, R. Villalobos-Valencia, T. Yamashita, B. Zurawski, E.C. de Bruin, L. Grinsted, C. D’Cruz, A. Foxley, Y.H. Park
Published in Annals of Oncology, December 2025
Background
Metastatic triple-negative breast cancer (mTNBC) remains one of the most aggressive breast cancer subtypes, characterized by rapid progression, limited targeted treatment options, and poor long-term outcomes. Unlike hormone receptor–positive or HER2-positive disease, TNBC lacks established oncogenic drivers that can be routinely targeted in clinical practice. Genomic analyses have consistently demonstrated frequent activation of the PI3K/AKT/mTOR signaling pathway in TNBC, often driven by alterations in PIK3CA, AKT1, or loss of PTEN, providing a strong biological rationale for AKT inhibition in this setting.
Capivasertib is an oral, selective pan-AKT inhibitor that previously demonstrated promising signals of efficacy when combined with paclitaxel in the randomized phase II PAKT trial, including improvements in progression-free survival (PFS) and overall survival (OS), particularly in biomarker-selected populations. The global phase III CAPItello-290 trial was therefore designed to definitively evaluate whether adding capivasertib to first-line paclitaxel could improve survival outcomes in patients with previously untreated mTNBC, both in the overall population and in tumors harboring PIK3CA/AKT1/PTEN alterations.
Methods
Eligible patients had histologically confirmed, previously untreated metastatic or locally advanced unresectable TNBC. Prior systemic therapy for metastatic disease was not allowed. Patients were required to have adequate organ function and ECOG performance status suitable for combination chemotherapy. Molecular alterations in PIK3CA, AKT1, and PTEN were assessed retrospectively using centralized molecular testing, allowing prespecified subgroup analyses without restricting enrollment.
The trial incorporated dual primary endpoints: overall survival in the overall population and overall survival in patients with PIK3CA/AKT1/PTEN-altered tumors. Key secondary endpoints included investigator-assessed PFS, objective response rate (ORR), and safety.
Study Design
CAPItello-290 was a randomized, double-blind, placebo-controlled, global phase III trial. Patients were randomized 1:1 to receive either:
- Paclitaxel 80 mg/m² administered on Day 1 of Weeks 1–3 of a 28-day cycle, plus capivasertib 400 mg orally twice daily on Days 2–5 of Weeks 1–3
- The same paclitaxel regimen plus matched placebo on the same intermittent schedule
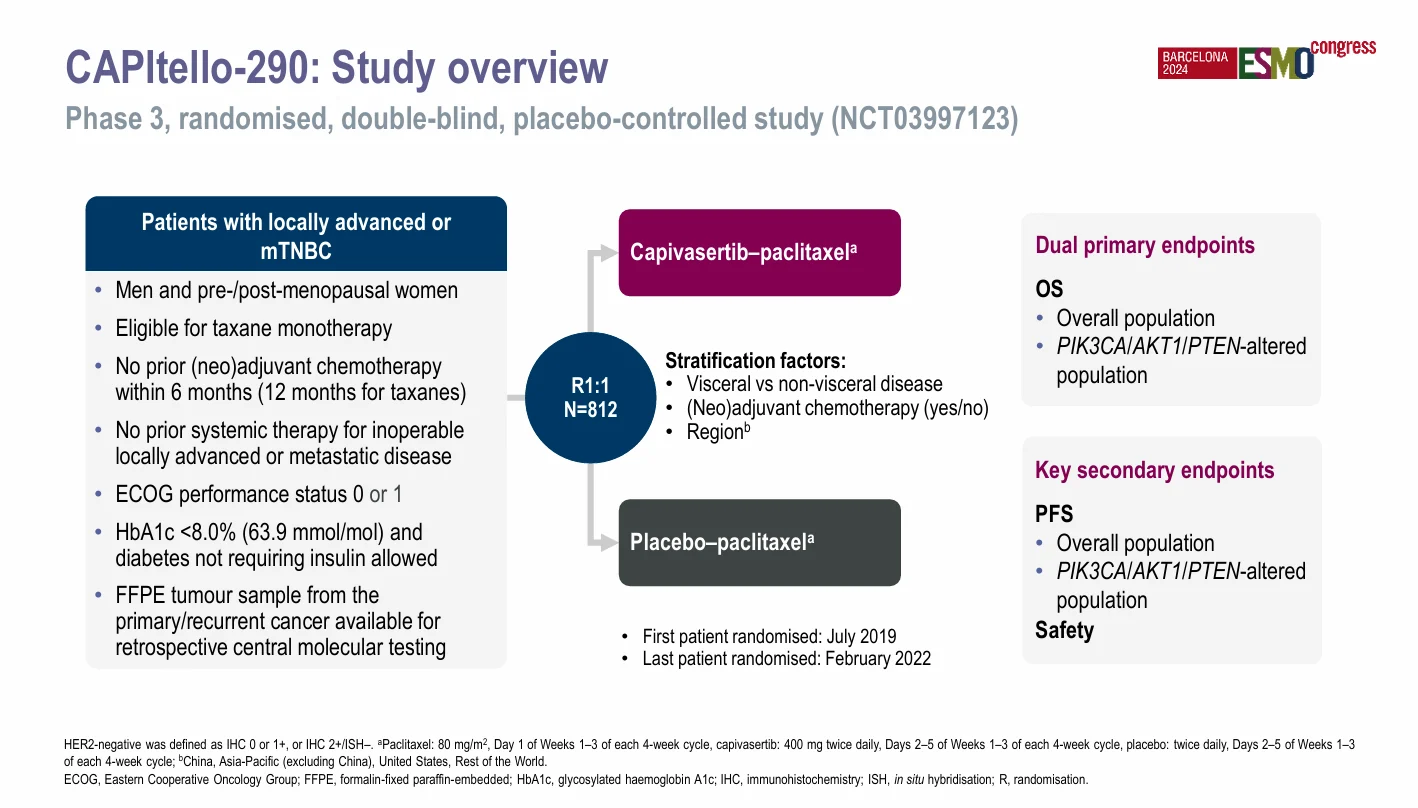
Treatment continued until disease progression, unacceptable toxicity, or withdrawal of consent. The intermittent dosing strategy for capivasertib was selected to mitigate on-target toxicities while maintaining pathway inhibition.
Results
Between July 2019 and February 2022, a total of 812 patients were randomized. PIK3CA/AKT1/PTEN alterations were identified in 30.7% of tumors, consistent with prior genomic datasets in TNBC.
At the final overall survival analysis (data cut-off March 18, 2024), the addition of capivasertib to paclitaxel did not meet the prespecified boundary for OS improvement. Median OS in the overall population was 17.7 months with capivasertib–paclitaxel versus 18.0 months with placebo–paclitaxel (hazard ratio [HR] 0.92, 95% CI 0.78–1.08; P = 0.3239).
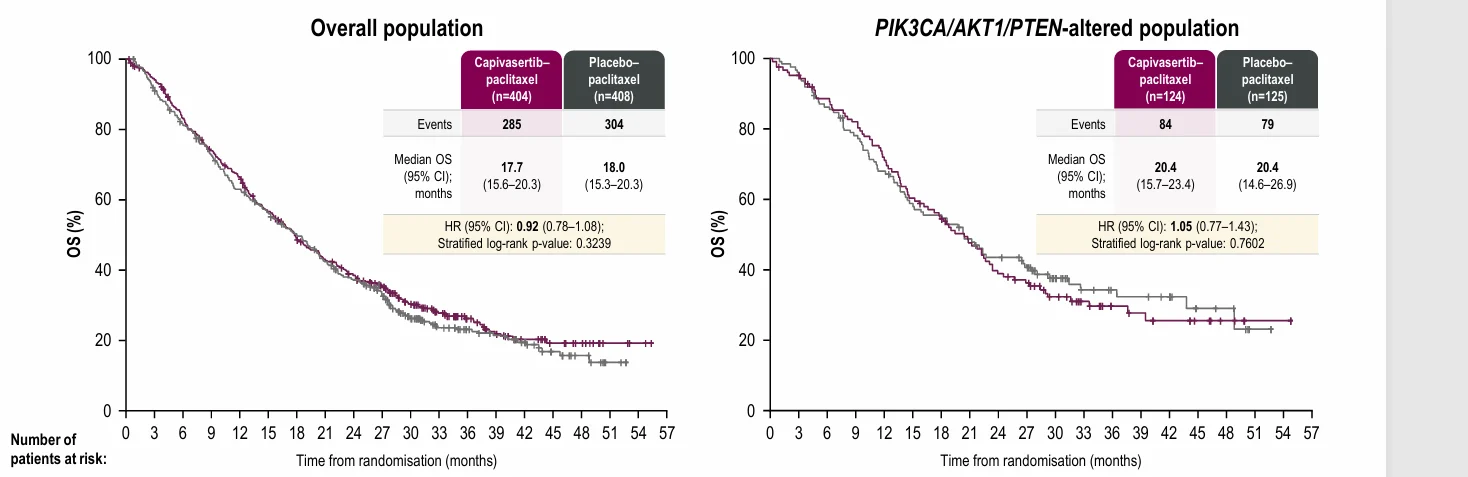
In the biomarker-defined subgroup, median OS was 20.4 months in both arms, with an HR of 1.05 (95% CI 0.77–1.43; P = 0.7602), indicating no survival advantage in tumors harboring PIK3CA/AKT1/PTEN alterations.In contrast, progression-free survival showed a numerical improvement favoring the capivasertib combination.
At the PFS data cut-off (May 25, 2022), median PFS in the overall population was 5.6 months with capivasertib–paclitaxel compared with 5.1 months for placebo–paclitaxel (HR 0.72, 95% CI 0.61–0.84). In patients with PIK3CA/AKT1/PTEN-altered tumors, median PFS was 7.5 months versus 5.6 months, respectively (HR 0.70, 95% CI 0.52–0.95), suggesting enhanced activity in this biologically defined subgroup.
Objective response rates also numerically favored the experimental arm, supporting antitumor activity of AKT inhibition when combined with chemotherapy, although these gains did not translate into a statistically significant OS benefit.
The safety profile of capivasertib–paclitaxel was generally manageable and consistent with prior experience. The most common grade ≥3 adverse event was diarrhea, occurring in 12.7% of patients in the capivasertib arm compared with 0.7% in the placebo arm. Treatment discontinuation due to adverse events occurred in 8.5% of patients receiving capivasertib–paclitaxel versus 4.9% with placebo–paclitaxel. Adverse events leading to death were reported in 4.2% of patients, underscoring the importance of vigilant toxicity monitoring in this population.
Key Findings
- The trial did not demonstrate an overall survival benefit with capivasertib plus paclitaxel in first-line mTNBC.
- Progression-free survival numerically improved, with a more pronounced effect in PIK3CA/AKT1/PTEN-altered tumors.
- The safety profile was consistent with known AKT inhibitor toxicities, with diarrhea as the most clinically relevant adverse event.
- Biomarker-selected patients showed greater PFS benefit, but this did not translate into OS improvement.
Conclusion
The CAPItello-290 trial provides a definitive evaluation of capivasertib plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer. While the combination demonstrated consistent numerical improvements in progression-free survival—particularly in tumors with PI3K/AKT pathway alterations—it failed to improve overall survival in either the overall or biomarker-defined populations.
These findings highlight the ongoing difficulty of translating pathway inhibition into durable survival gains in TNBC and reinforce the need for continued biomarker refinement and novel combination strategies to improve outcomes in this high-risk disease.
You can read the full article here.


