The CABATEN trial (GETNE-T1914) was a multicenter, basket Phase II study that investigated the combination of cabozantinib, a multikinase inhibitor (MKI), and atezolizumab, a PD-L1 immune checkpoint inhibitor (ICI), in patients with advanced and progressive endocrine malignancies. Rare tumors sucah as anaplastic thyroid cancer (ATC), adrenocortical carcinoma (ACC), and malignant pheochromocytoma/paraganglioma (PPGL) often have limited systemic treatment options and poor prognosis, with median overall survival ranging from 6 to 12 months in aggressive subtypes.
While MKIs have demonstrated efficacy in well-differentiated neuroendocrine tumors (NETs) and thyroid cancers, their benefit in highly aggressive endocrine tumors has been less consistent. Similarly, ICIs have shown limited single-agent activity in indolent NETs but potential utility in aggressive histologies such as ATC and ACC. Preclinical and clinical data suggest that combining MKIs with ICIs may overcome intrinsic and acquired resistance mechanisms, thereby improving antitumor efficacy.
Title: Cabozantinib plus Atezolizumab in Advanced, Progressive Endocrine Malignancies: A multi-cohort, Basket, Phase II Trial (CABATEN/GETNE-T1914)
Published in Clinical Cancer Research, September 2025
Authors: Jaume Capdevila, Jorge Hernando, Javier Molina-Cerrillo, Marta Benavent Viñuales, Rocio Garcia-Carbonero, Alex Teulé, Ana Custodio, Paula Jimenez-Fonseca, Carlos Lopez, Cinta Hierro, Alberto Carmona-Bayonas, Vicente Alonso, Marta Llanos, Isabel Sevilla, Alejandro Garcia-Alvarez, Teresa Alonso-Gordoa, Inmaculada Gallego Jiménez, Beatriz Antón-Pascual, Andrea Modrego Sánchez, Enrique Grande
Background
Endocrine and neuroendocrine malignancies, including anaplastic thyroid cancer (ATC), adrenocortical carcinoma (ACC), pheochromocytoma/paraganglioma (PPGL), and gastroenteropancreatic neuroendocrine tumors (GEP-NETs), represent a heterogeneous group of rare cancers with limited systemic treatment options. Prognosis remains especially poor for ATC and ACC, where median overall survival (OS) is typically 6–12 months despite standard therapies.
Multikinase inhibitors (MKIs) such as cabozantinib have shown activity in well-differentiated tumors, and immune checkpoint inhibitors (ICIs) like atezolizumab have demonstrated efficacy in other aggressive cancers. Combining MKIs with ICIs has shown synergistic potential across tumor types.
The CABATEN trial was designed to evaluate whether this combination could improve outcomes in advanced, progressive endocrine malignancies that are refractory to standard therapies.
Methods
CABATEN (EudraCT: 2019-002279-32, NCT04400474) was a prospective, open-label, multicenter, multi-cohort Phase II trial conducted across 15 hospitals in Spain. Eligible patients were adults (>18 years) with advanced or refractory endocrine and neuroendocrine neoplasms, an ECOG performance status of 0–1, and adequate organ function.
Patients received atezolizumab 1200 mg intravenously every three weeks plus oral cabozantinib 40 mg daily. Dose reductions to 20 mg/day were allowed in cases of unacceptable toxicity. Treatment continued until progression, unacceptable toxicity, withdrawal, or death. Tumor response was assessed by RECIST 1.1 every 12 weeks.
Study Design
The study used a Simon two-stage optimal design. Six cohorts were included:
- Lung well-differentiated NETs (lungNET, n=9)
- Anaplastic thyroid cancer (ATC, n=14)
- Adrenocortical carcinoma (ACC, n=24)
- Malignant pheochromocytoma/paraganglioma (PPGL, n=13)
- Well-differentiated GEP-NET (n=24)
- Grade 3 extrapulmonary neuroendocrine neoplasms (G3 EP-NEN, n=9)
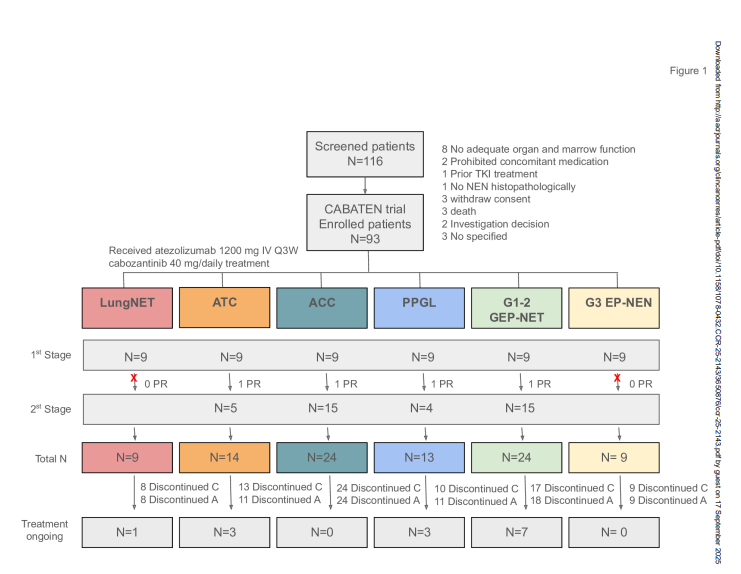
The primary endpoint was overall response rate (ORR). Secondary endpoints included duration of response (DoR), progression-free survival (PFS), overall survival (OS), safety, and quality of life (QoL).
Results
Between October 2020 and December 2022, 93 patients were enrolled. Median age ranged from 48 to 63 years depending on cohort. Most patients were heavily pretreated: 92% of those with ACC had prior chemotherapy, and 83% of those with GEP-NET had prior somatostatin analogs.
Efficacy
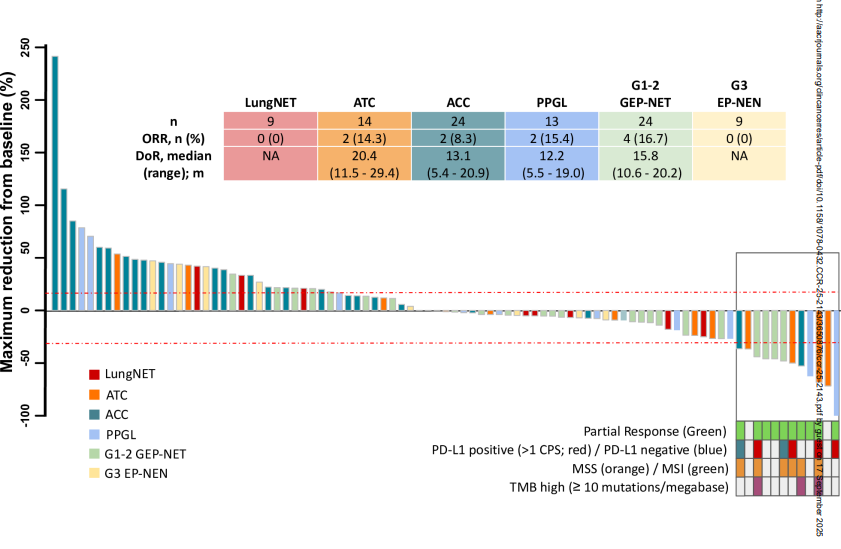
- ATC: ORR 14% (2/14 patients). Median DoR was 20.4 months. Median OS reached 6.1 months, with a 12-month OS rate of 48%. Notably, BRAF wild-type ATC achieved an ORR of 20%, while BRAF-mutated ATC showed no responses.
- ACC: ORR 8% (2/24 patients). Median DoR was 13.1 months. Median OS was 13.5 months, with a 12-month OS rate of 58%. Outcomes were worse in patients with Cushing syndrome.
- PPGL: ORR 15% (2/13 patients). Median DoR was 12.2 months. Median OS was 26.7 months, with a 12-month OS rate of 62%.
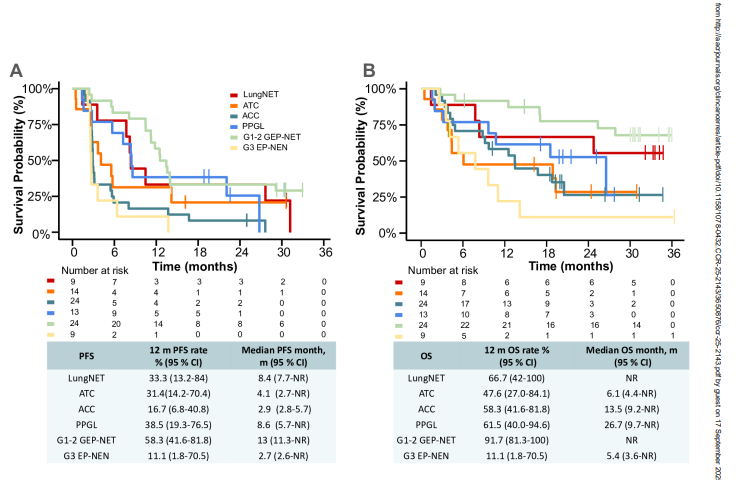
- GEP-NET: ORR 17% (4/24 patients). Median DoR was 15.8 months. Median OS was not reached; the 12-month OS rate was 92%.
- LungNET and G3 EP-NEN: No objective responses observed. Median OS was not reached in lungNET, while G3 EP-NEN showed median OS of only 5.4 months with an 11% 12-month OS rate.
- Median PFS varied by cohort: 4.1 months (ATC), 2.9 months (ACC), 8.6 months (PPGL), 13 months (GEP-NET), 8.4 months (lungNET), and 2.7 months (G3 EP-NEN).
Safety
The safety profile was manageable and consistent with known effects of VEGF inhibitors and ICIs.
- Most frequent treatment-related adverse events (AEs): fatigue (60%), diarrhea (43%), elevated AST (32%), elevated ALT (28%), oral mucositis (27%), nausea (26%), and hypertension (26%).
- Grade ≥3 AEs: fatigue (10%), ALT increase (9%), neutropenia (7%), and hypertension (5%).
- Serious AEs occurred in 46% of patients, with two treatment-related deaths (acute pancreatitis and cerebral artery stroke).
Dose reductions of cabozantinib to 20 mg/day were required in 45% of patients, but efficacy outcomes were not significantly affected by dose intensity.
Key Findings
- The combination of cabozantinib plus atezolizumab demonstrated promising efficacy in ATC and ACC, where therapeutic options are scarce.
- ORR was modest across cohorts (ranging from 8–17%), but responses were durable, with DoR exceeding 12 months in most responding patients.
- ATC patients with BRAF wild-type tumors and ACC patients without hypercortisolism derived the most benefit.
- LungNET and G3 EP-NEN cohorts showed no responses, suggesting limited role in these subtypes.
- Safety was manageable, with toxicity consistent with prior cabozantinib or atezolizumab studies.
Key Takeaway Messages
- For ATC and ACC, cabozantinib plus atezolizumab provides a feasible treatment option with durable responses and encouraging OS outcomes, especially in pretreated patients.
- For GEP-NET and PPGL, activity was modest and not clearly superior to monotherapy with MKIs.
- For lungNET and G3 EP-NEN, the regimen showed no meaningful benefit, highlighting the need for alternative strategies.\Further trials should focus on identifying predictive biomarkers (e.g., PD-L1 expression, BRAF status, cortisol secretion) to refine patient selection.
Conclusion
The CABATEN trial provides important prospective evidence supporting the combination of cabozantinib and atezolizumab in selected aggressive endocrine malignancies. While overall response rates were modest, the durability of responses and encouraging survival outcomes in anaplastic thyroid cancer and adrenocortical carcinoma suggest clinical relevance for patients with few effective options.
The activity observed in malignant pheochromocytoma/paraganglioma and gastroenteropancreatic NETs was limited, and no benefit was seen in lungNET or G3 EP-NEN, underscoring the heterogeneity of response across endocrine neoplasms. The manageable safety profile reinforces the feasibility of this regimen. Moving forward, biomarker-driven patient selection will be critical to optimize efficacy and identify those most likely to benefit from this therapeutic strategy.
You can read the full article here.


